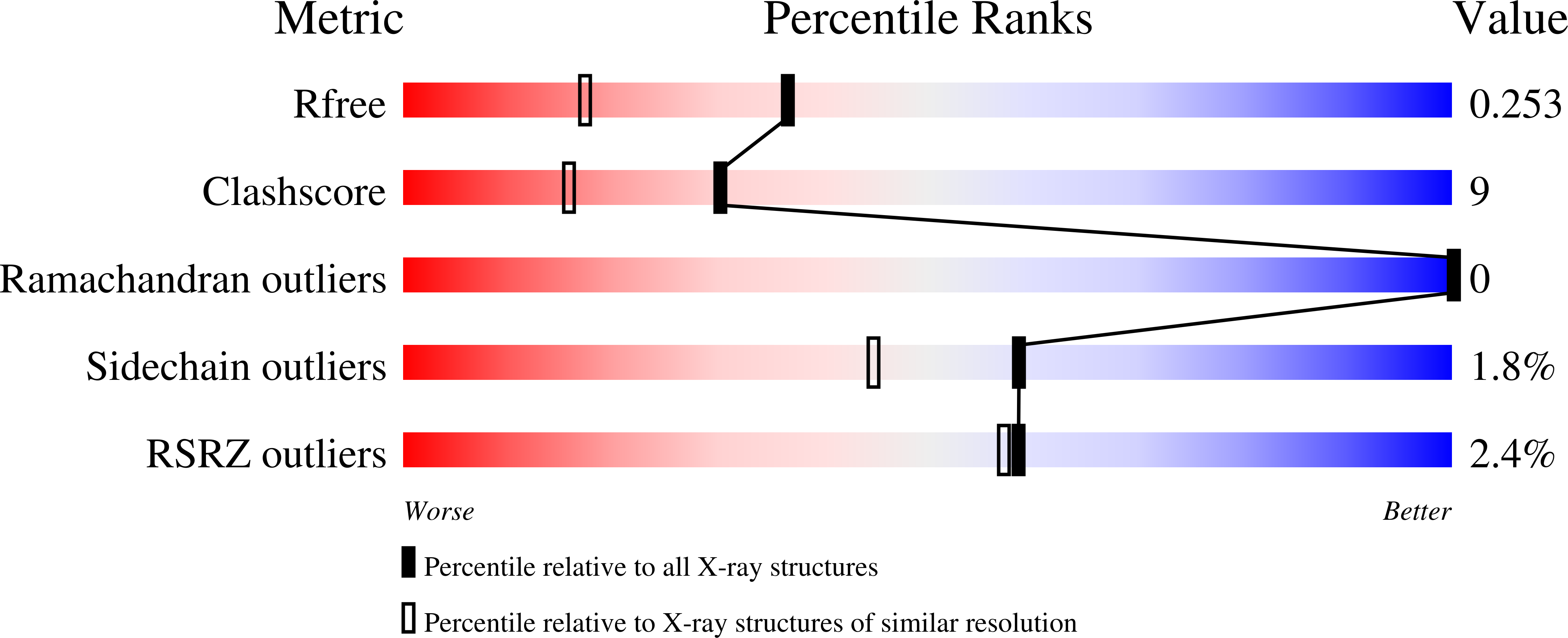
Crystal structures of Cif from bacterial pathogens Photorhabdus luminescens and Burkholderia pseudomallei.
Crow, A., Race, P.R., Jubelin, G., Varela Chavez, C., Escoubas, J.M., Oswald, E., Banfield, M.J.(2009) PLoS One 4: e5582-e5582
- PubMed: 19440549
- DOI: https://doi.org/10.1371/journal.pone.0005582
- Primary Citation of Related Structures:
3GQJ, 3GQM - PubMed Abstract:
A pre-requisite for bacterial pathogenesis is the successful interaction of a pathogen with a host. One mechanism used by a broad range of Gram negative bacterial pathogens is to deliver effector proteins directly into host cells through a dedicated type III secretion system where they modulate host cell function. The cycle inhibiting factor (Cif) family of effector proteins, identified in a growing number of pathogens that harbour functional type III secretion systems and have a wide host range, arrest the eukaryotic cell cycle. Here, the crystal structures of Cifs from the insect pathogen/nematode symbiont Photorhabdus luminescens (a gamma-proteobacterium) and human pathogen Burkholderia pseudomallei (a beta-proteobacterium) are presented. Both of these proteins adopt an overall fold similar to the papain sub-family of cysteine proteases, as originally identified in the structure of a truncated form of Cif from Enteropathogenic E. coli (EPEC), despite sharing only limited sequence identity. The structure of an N-terminal region, referred to here as the 'tail-domain' (absent in the EPEC Cif structure), suggests a surface likely to be involved in host-cell substrate recognition. The conformation of the Cys-His-Gln catalytic triad is retained, and the essential cysteine is exposed to solvent and addressable by small molecule reagents. These structures and biochemical work contribute to the rapidly expanding literature on Cifs, and direct further studies to better understand the molecular details of the activity of these proteins.
Organizational Affiliation:
Department of Biological Chemistry, John Innes Centre, Norwich, United Kingdom.