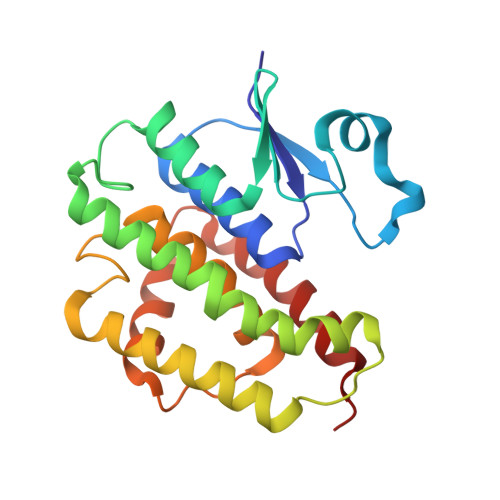
Crystallographic survey of active sites of an unclassified glutathione transferase from Bombyx mori
Kakuta, Y., Usuda, K., Nakashima, T., Kimura, M., Aso, Y., Yamamoto, K.(2011) Biochim Biophys Acta 1810: 1355-1360
- PubMed: 21756974
- DOI: https://doi.org/10.1016/j.bbagen.2011.06.022
- Primary Citation of Related Structures:
3AY8 - PubMed Abstract:
Glutathione transferase (GST) catalyzes a major step in the xenobiotic detoxification pathway. We previously identified a novel, unclassified GST that is upregulated in an insecticide-resistant silkworm (Bombyx mori) upon insecticide exposure. Here, we sought to further characterize this GST, bmGSTu, by solving and refining its crystal structure and identifying its catalytic residues.
Organizational Affiliation:
Faculty of Agriculture, Kyushu University Graduate School, 6-10-1 Hakozaki, Higashi-ku, Fukuoka 812-8581, Japan.