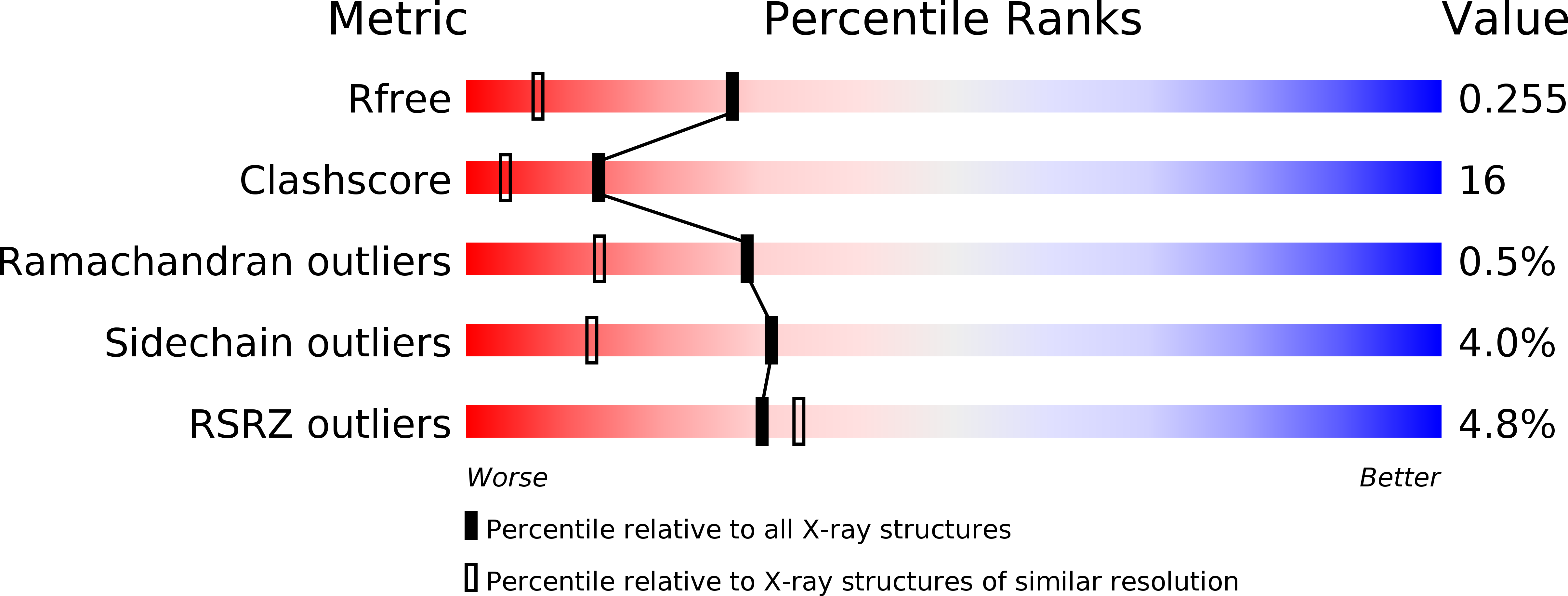
Thermodynamic Characterization of the Interaction between Prefoldin and Group II Chaperonin
Sahlan, M., Zako, T., Tai, P.T., Ohtaki, A., Noguchi, K., Maeda, M., Miyatake, H., Dohmae, N., Yohda, M.(2010) J Mol Biol 399: 628-636
- PubMed: 20434454
- DOI: https://doi.org/10.1016/j.jmb.2010.04.046
- Primary Citation of Related Structures:
3AEI - PubMed Abstract:
Prefoldin (PFD) is a hexameric chaperone that captures a protein substrate and transfers it to a group II chaperonin (CPN) to complete protein folding. We have studied the interaction between PFD and CPN using those from a hyperthermophilic archaeon, Thermococcus strain KS-1 (T. KS-1). In this study, we determined the crystal structure of the T. KS-1 PFDbeta2 subunit and characterized the interactions between T. KS-1 CPNs (CPNalpha and CPNbeta) and T. KS-1 PFDs (PFDalpha1-beta1 and PFDalpha2-beta2). As predicted from its amino acid sequence, the PFDbeta2 subunit conforms to a structure similar to those of the PFDbeta1 subunit and the Pyrococcus horikoshii OT3 PFDbeta subunit, with the exception of the tip of its coiled-coil domain, which is thought to be the CPN interaction site. The interactions between T. KS-1 CPNs and PFDs (CPNalpha and PFDalpha1-beta1; CPNalpha and PFDalpha2-beta2; CPNbeta and PFDalpha1-beta1; and CPNbeta and PFDalpha2-beta2) were analyzed using the Biacore T100 system at various temperatures ranging from 20 to 45 degrees C. The affinities between PFDs and CPNs increased with an increase in temperature. The thermodynamic parameters calculated from association constants showed that the interaction between PFD and CPN is entropy driven. Among the four combinations of PFD-CPN interactions, the entropy difference in binding between CPNbeta and PFDalpha2-beta2 was the largest, and affinity significantly increased at higher temperatures. Considering that expression of PFDalpha2-beta2 and CPNbeta subunit is induced upon heat shock, our results suggest that PFDalpha1-beta1 is a general PFD for T. KS-1 CPNs, whereas PFDalpha2-beta2 is specific for CPNbeta.
Organizational Affiliation:
Department of Biotechnology and Life Science, Tokyo University of Agriculture and Technology, 2-24-16 Naka-cho, Koganei-shi, Tokyo 184-8588, Japan.