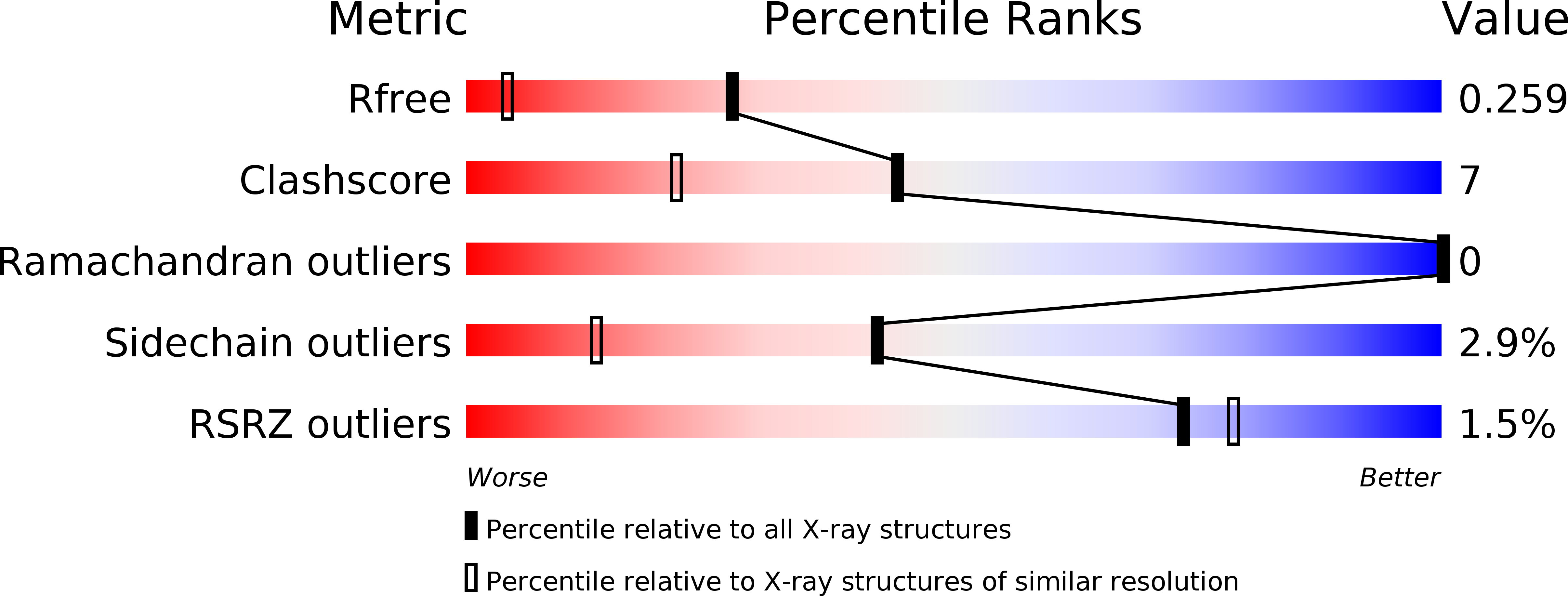
Structure of a Trypanosomatid Mitochondrial Cytochrome C with Heme Attached Via Only One Thioether Bond and Implications for the Substrate Recognition Requirements of Heme Lyase.
Fulop, V., Sam, K.A., Ferguson, S.J., Ginger, M.L., Allen, J.W.A.(2009) FEBS J 276: 2822
- PubMed: 19459937
- DOI: https://doi.org/10.1111/j.1742-4658.2009.07005.x
- Primary Citation of Related Structures:
2YK3 - PubMed Abstract:
The principal physiological role of mitochondrial cytochrome c is electron transfer during oxidative phosphorylation. c-Type cytochromes are almost always characterized by covalent attachment of heme to protein through two thioether bonds between the heme vinyl groups and the thiols of cysteine residues in a Cys-Xxx-Xxx-Cys-His motif. Uniquely, however, members of the evolutionarily divergent protist phylum Euglenozoa, which includes Trypanosoma and Leishmania species, have mitochondrial cytochromes c with heme attached through only one thioether bond [to an (A/F)XXCH motif]; the implications of this for the cytochrome structures are unclear. Here we present the 1.55 A resolution X-ray crystal structure of cytochrome c from the trypanosomatid Crithidia fasciculata. Despite the fundamental difference in heme attachment and in the cytochrome c biogenesis machinery of the Euglenozoa, the structure is remarkably similar to that of typical (CXXCH) mitochondrial cytochromes c, both in overall fold and, other than the missing thioether bond, in the details of the heme attachment. Notably, this similarity includes the stereochemistry of the covalent heme attachment to the protein. The structure has implications for the maturation of c-type cytochromes in the Euglenozoa; it also hints at a distinctive redox environment in the mitochondrial intermembrane space of trypanosomes. Surprisingly, Saccharomyces cerevisiae cytochrome c heme lyase (the yeast cytochrome c biogenesis system) cannot efficiently mature Trypanosoma brucei cytochrome c or a CXXCH variant when expressed in the cytoplasm of Escherichia coli, despite their great structural similarity to yeast cytochrome c, suggesting that heme lyase requires specific recognition features in the apocytochrome.
Organizational Affiliation:
Department of Biological Sciences, University of Warwick, Coventry, UK.