The Structure of the Fyr Domain of Transforming Growth Factor Beta Regulator 1 (Tbrg1)(Pna)
Garcia-Alai, M.M., Allen, M.D., Joerger, A.C., Bycroft, M.(2010) Protein Sci 19: 1432
- PubMed: 20506279
- DOI: https://doi.org/10.1002/pro.404
- Primary Citation of Related Structures:
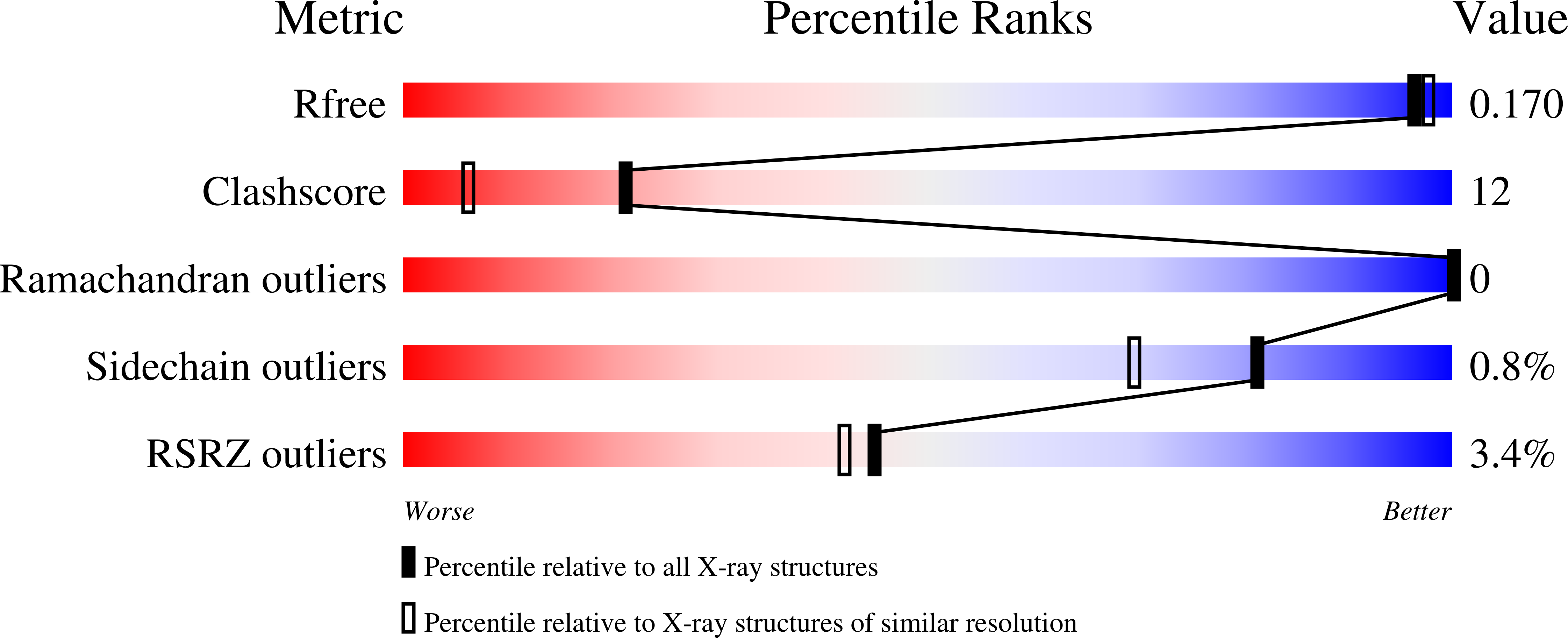
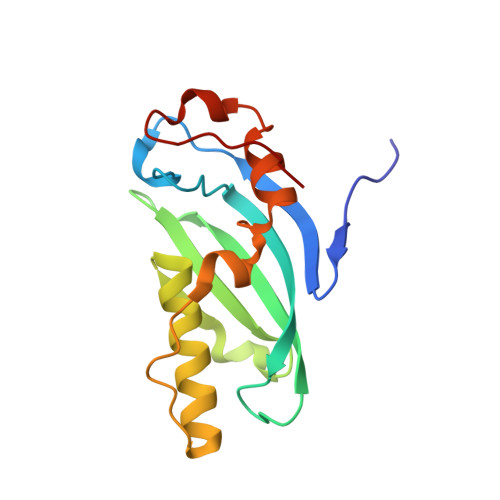
2WZO - PubMed Abstract:
Many chromatin-associated proteins contain two sequence motifs rich in phenylalanine/tyrosine residues of unknown function. These so-called FYRN and FYRC motifs are also found in transforming growth factor beta regulator 1 (TBRG1)/nuclear interactor of ARF and MDM2 (NIAM), a growth inhibitory protein that also plays a role in maintaining chromosomal stability. We have solved the structure of a fragment of TBRG1, which encompasses both of these motifs. The FYRN and FYRC regions each form part of a single folded module (the FYR domain), which adopts a novel alpha + beta fold. Proteins such as the histone H3K4 methyltransferases trithorax and mixed lineage leukemia (MLL), in which the FYRN and FYRC regions are separated by hundreds of amino acids, are expected to contain FYR domains with a large insertion between two of the strands of the beta-sheet.
Organizational Affiliation:
MRC Centre for Protein Engineering, Hills Road, Cambridge CB2 0QH, United Kingdom.