Neuroplastin-55 Binds to and Signals Through the Fibroblast Growth Factor Receptor
Owczarek, S., Kiryushko, D., Larsen, M.H., Kastrup, J.S., Gajhede, M., Sandi, C., Berezin, V., Bock, E., Soroka, V.(2010) FASEB J 24: 1139
- PubMed: 19952283
- DOI: https://doi.org/10.1096/fj.09-140509
- Primary Citation of Related Structures:
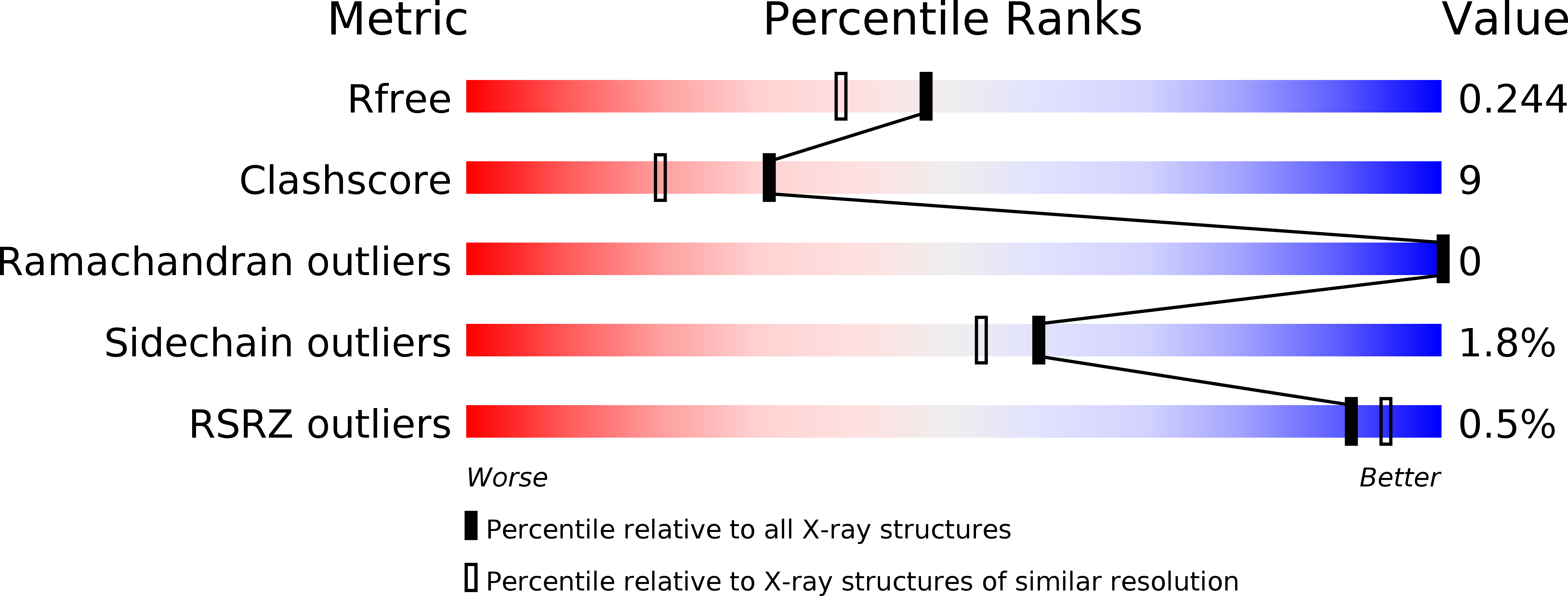
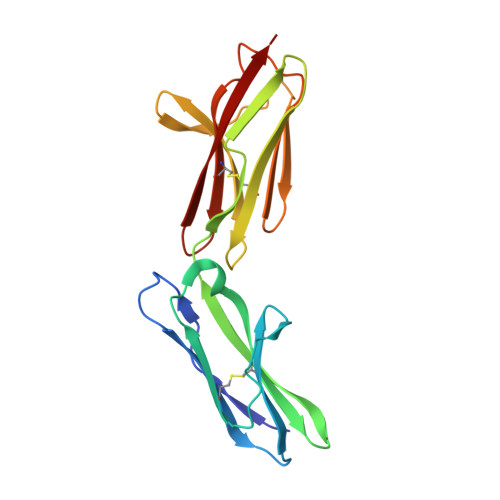
2WV3 - PubMed Abstract:
Neuroplastin (Np) is a glycoprotein belonging to the immunoglobulin superfamily of cell adhesion molecules (CAMs) and existing in two isoforms, Np55 and Np65, named according to their molecular weights. The extracellular part of Np65 contains three immunoglobulin (Ig)-like modules (Ig1, Ig2, and Ig3), whereas Np55 lacks the Ig1 module. Of these two isoforms, only Np65 is involved in homophilic interactions resulting in cell adhesion, whereas the role of Np55 is poorly understood. The present study reports for the first time the crystal structure of the ectodomain of Np55 at 1.95-A resolution and demonstrates that Np55 binds to and activates the fibroblast growth factor receptor 1 (FGFR1). Furthermore, we identify a sequence motif in the Ig2 module of Np55 interacting with FGFR1 and show that a synthetic peptide encompassing this motif, termed narpin, binds to and activates FGFR1. We show that both Np55 and the narpin peptide induce neurite outgrowth through FGFR1 activation and that Np55 increases synaptic calcium concentration in an FGFR1-dependent manner. Moreover, we demonstrate that narpin has an antidepressive-like effect in rats subjected to the forced swim test, suggesting that Np55-induced signaling may be involved in synaptic plasticity in vivo. Owczarek, S., Kiryushko, D., Larsen, M. H., Kastrup, J. S., Gajhede, M., Sandi, C., Berezin, V., Bock, E., Soroka, V. Neuroplastin-55 binds to and signals through the fibroblast growth factor receptor.
Organizational Affiliation:
Protein Laboratory, Department of Neuroscience and Pharmacology, Panum Institute, University of Copenhagen, Blegdamsvej 3C, Bldg. 24.2, 2200 Copenhagen, Denmark. sylwia@sund.ku.dk