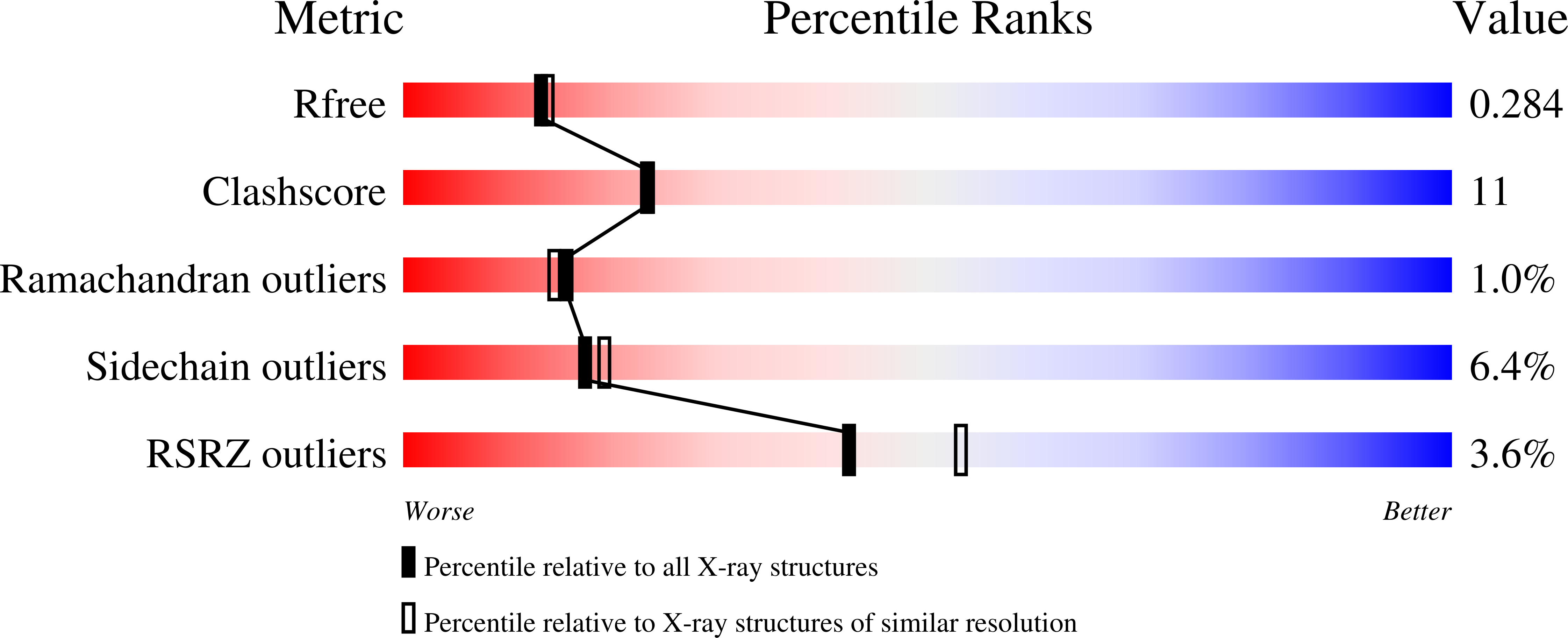
Anatomy of a Simple Acyl Intermediate in Enzyme Catalysis: Combined Biophysical and Modeling Studies on Ornithine Acetyl Transferase.
Iqbal, A., Clifton, I.J., Bagonis, M., Kershaw, N.J., Domene, C., Claridge, T.D., Wharton, C.W., Schofield, C.J.(2009) J Am Chem Soc 131: 749
- PubMed: 19105697
- DOI: https://doi.org/10.1021/ja807215u
- Primary Citation of Related Structures:
2VZK - PubMed Abstract:
Acyl-enzyme complexes are intermediates in reactions catalyzed by many hydrolases and related enzymes which employ nucleophilic catalysis. However, most of the reported structural data on acyl-enzyme complexes has been acquired under noncatalytic conditions. Recent IR analyses have indicated that some acyl-enzyme complexes may be more flexible than most crystallographic analyses have implied. OAT2 is a member of the N-terminal nucleophile (Ntn) hydrolase enzyme superfamily and catalyzes the reversible transfer of an acetyl group between the alpha-amino groups of ornithine and glutamate in a mechanism proposed to involve an acyl-enzyme complex. We have carried out biophysical analyses on ornithine acetyl transferase (OAT2), both in solution and in the crystalline state. Mass spectrometric studies identified Thr-181 as the residue acetylated during OAT2 catalysis; (13)C NMR analyses implied the presence of an acyl-enzyme complex in solution. Crystallization of OAT2 in the presence of N-alpha-acetyl-L-glutamate led to a structure in which Thr-181 was acetylated; the carbonyl oxygen of the acyl-enzyme complex was located in an oxyanion hole and positioned to hydrogen bond with the backbone amide NH of Gly-112 and the alcohol of Thr-111. While the crystallographic analyses revealed only one structure, IR spectroscopy demonstrated the presence of two distinct acyl-enzyme complex structures with carbonyl stretching frequencies at 1691 and 1701 cm(-1). Modeling studies implied two possible acyl-enzyme complex structures, one of which correlates with that observed in the crystal structure and with the 1691 cm(-1) IR absorption. The second acyl-enzyme complex structure, which has only a single oxyanion hole hydrogen bond, is proposed to give rise to the 1701 cm(-1) IR absorption. The two acyl-enzyme complex structures can interconvert by movement of the Thr-111 side-chain alcohol hydrogen away from the oxyanion hole to hydrogen bond with the backbone carbonyl of the acylated residue, Thr-181. Overall, the results reveal that acyl-enzyme complex structures may be more dynamic than previously thought and support the use of a comprehensive biophysical and modeling approach in studying such intermediates.
Organizational Affiliation:
Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA, UK.