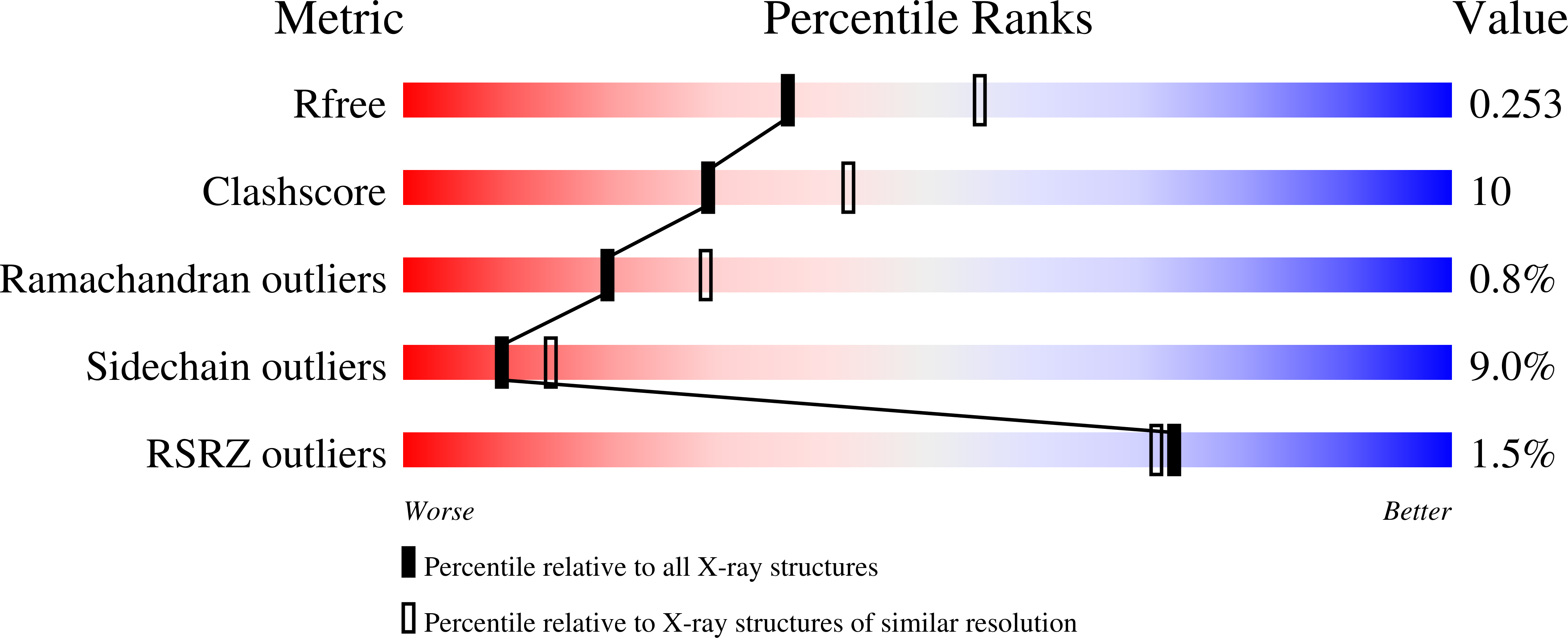
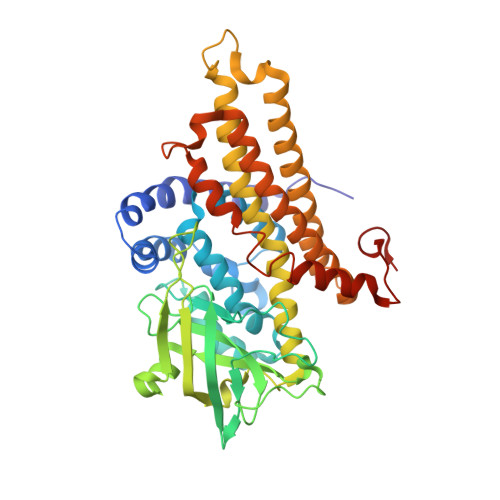
Mechanistic and structural analyses of the roles of Arg409 and Asp402 in the reaction of the flavoprotein nitroalkane oxidase.
Fitzpatrick, P.F., Bozinovski, D.M., Heroux, A., Shaw, P.G., Valley, M.P., Orville, A.M.(2007) Biochemistry 46: 13800-13808
- PubMed: 17994768
- DOI: https://doi.org/10.1021/bi701557k
- Primary Citation of Related Structures:
2REH, 2ZAF - PubMed Abstract:
The flavoprotein nitroalkane oxidase (NAO) catalyzes the oxidation of primary and secondary nitroalkanes to the corresponding aldehydes and ketones. The enzyme is a homologue of acyl-CoA dehydrogenase. Asp402 in NAO has been proposed to be the active site base responsible for removing the substrate proton in the first catalytic step; structurally it corresponds to the glutamate which acts as the base in medium chain acyl-CoA dehydrogenase. In the active site of NAO, the carboxylate of Asp402 forms an ionic interaction with the side chain of Arg409. The R409K enzyme has now been characterized kinetically and structurally. The mutation results in a decrease in the rate constant for proton abstraction of 100-fold. Analysis of the three-dimensional structure of the R409K enzyme, determined by X-ray crystallography to a resolution of 2.65 A, shows that the critical structural change is an increase in the distance between the carboxylate of Asp402 and the positively charged nitrogen in the side chain of the residue at position 409. The D402E mutation results in a smaller decrease in the rate constant for proton abstraction of 18-fold. The structure of the D402E enzyme, determined at 2.4 A resolution, shows that there is a smaller increase in the distance between Arg409 and the carboxylate at position 402, and the interaction of this residue with Ser276 is perturbed. These results establish the critical importance of the interaction between Asp402 and Arg409 for proton abstraction by nitroalkane oxidase.
Organizational Affiliation:
Department of Biochemistry and Biophysics, Texas A&M University, College Station, Texas 77843-2128, USA.