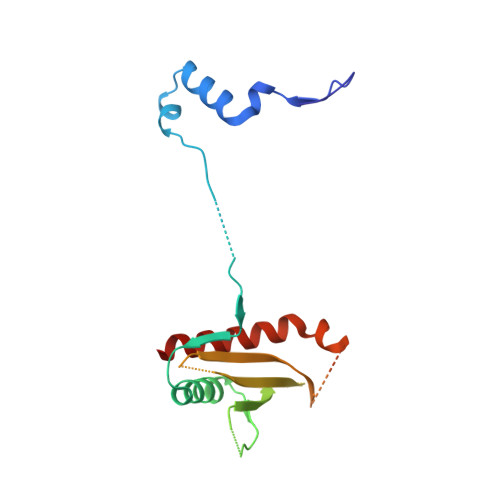
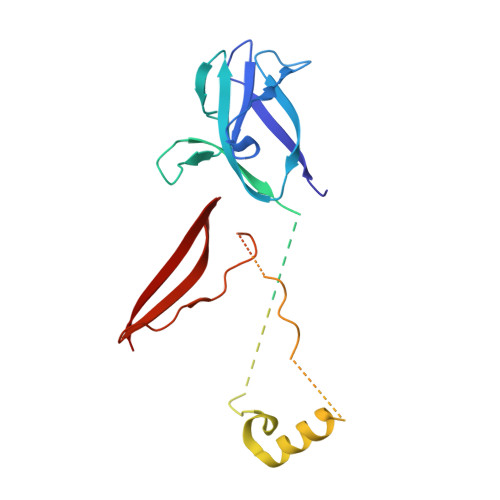
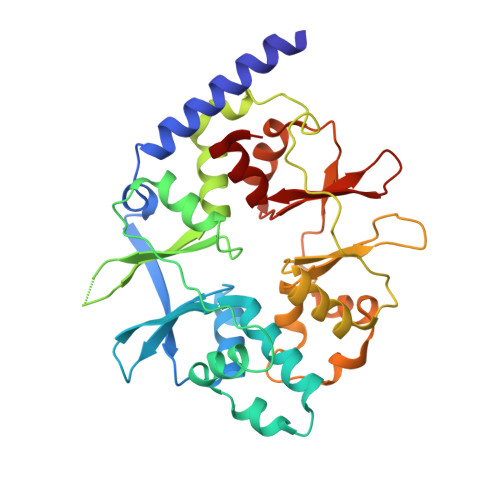
Crystal structure of the heterotrimer core of Saccharomyces cerevisiae AMPK homologue SNF1.
Amodeo, G.A., Rudolph, M.J., Tong, L.(2007) Nature 449: 492-495
- PubMed: 17851534
- DOI: https://doi.org/10.1038/nature06127
- Primary Citation of Related Structures:
2QLV - PubMed Abstract:
AMP-activated protein kinase (AMPK) is a central regulator of energy homeostasis in mammals and is an attractive target for drug discovery against diabetes, obesity and other diseases. The AMPK homologue in Saccharomyces cerevisiae, known as SNF1, is essential for responses to glucose starvation as well as for other cellular processes, although SNF1 seems to be activated by a ligand other than AMP. Here we report the crystal structure at 2.6 A resolution of the heterotrimer core of SNF1. The ligand-binding site in the gamma-subunit (Snf4) has clear structural differences from that of the Schizosaccharomyces pombe enzyme, although our crystallographic data indicate that AMP can also bind to Snf4. The glycogen-binding domain in the beta-subunit (Sip2) interacts with Snf4 in the heterotrimer but should still be able to bind carbohydrates. Our structure is supported by a large body of biochemical and genetic data on this complex. Most significantly, the structure reveals that part of the regulatory sequence in the alpha-subunit (Snf1) is sequestered by Snf4, demonstrating a direct interaction between the alpha- and gamma-subunits and indicating that our structure may represent the heterotrimer core of SNF1 in its activated state.
Organizational Affiliation:
Department of Biological Sciences, Columbia University, New York, New York 10027, USA.