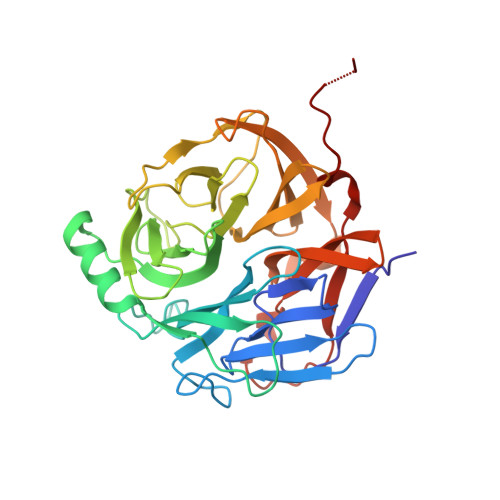
Crystal Structure of the Major Malassezia sympodialis Allergen Mala s 1 Reveals a beta-Propeller Fold: A Novel Fold Among Allergens.
Vilhelmsson, M., Zargari, A., Crameri, R., Rasool, O., Achour, A., Scheynius, A., Hallberg, B.M.(2007) J Mol Biol 369: 1079-1086
- PubMed: 17481656
- DOI: https://doi.org/10.1016/j.jmb.2007.04.009
- Primary Citation of Related Structures:
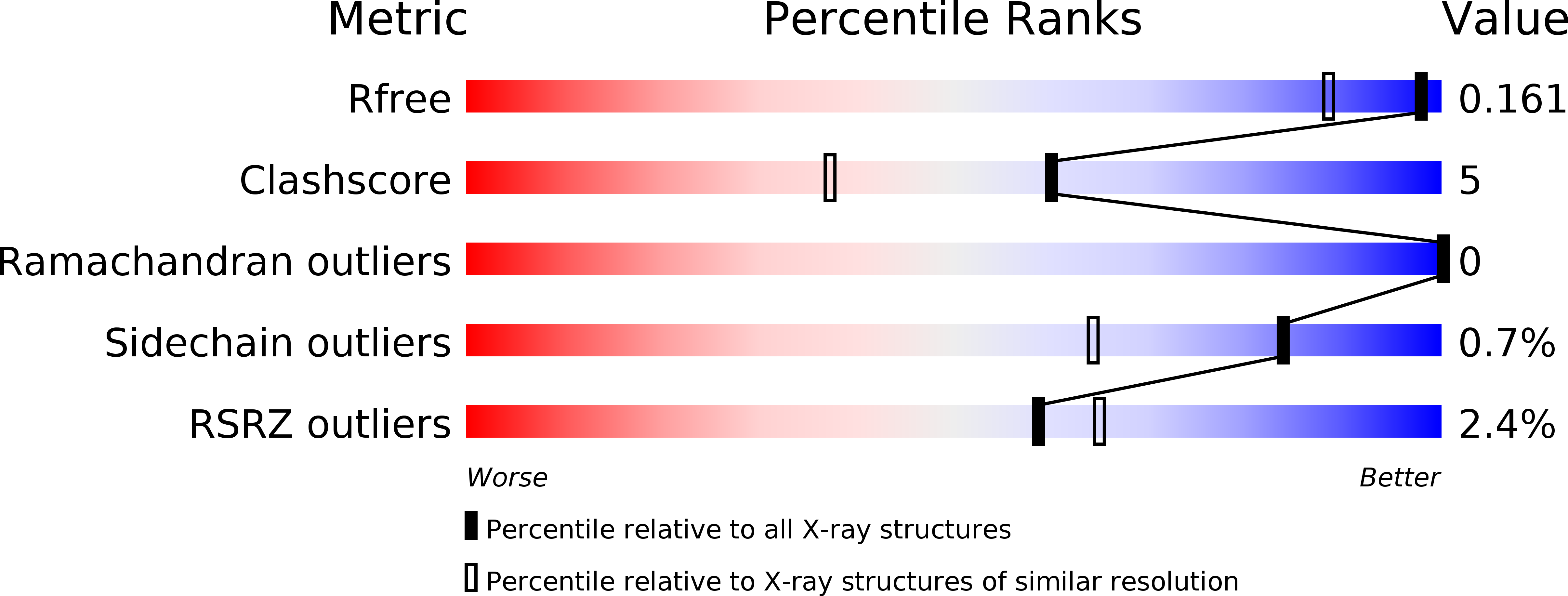
2P9W - PubMed Abstract:
Atopic eczema (AE) is a chronic inflammatory disease in which genetic predisposition and environmental factors such as microorganisms contribute to the symptoms. The yeast Malassezia Sympodialis, part of the normal human cutaneous flora, can act as an allergen eliciting specific IgE and T-cell reactivity in patients with AE. The major M. sympodialis allergen Mala s 1 is localized mainly in the yeast cell wall and exposed on the cell surface. Interestingly, Mala s 1 does not exhibit any significant sequence homology to known proteins. Here we present the crystal structure of Mala s 1 determined by single-wavelength anomalous dispersion techniques using selenomethionine-substituted Mala s 1. Mala s 1 folds into a 6-fold beta-propeller, a novel fold among allergens. The putative active site of Mala s 1 overlaps structurally to putative active sites in potential homologues, Q4P4P8 and Tri 14, from the plant parasites Ustilago maydis and Gibberella zeae, respectively. This resemblance suggests that Mala s 1 and the parasite proteins may have similar functions. In addition, we show that Mala s 1 binds to the phosphoinositides (PI) PI(3)P, PI(4)P, and PI(5)P, lipids possibly playing a role in the localization of Mala s 1 to the cell surface. The crystal structure of Mala s 1 will provide insights into the role of this major allergen in the host-microbe interactions and induction of an allergic response in AE.
Organizational Affiliation:
Department of Medicine, Clinical Allergy Research Unit, Karolinska Institutet and Karolinska University Hospital Solna, Stockholm, Sweden.