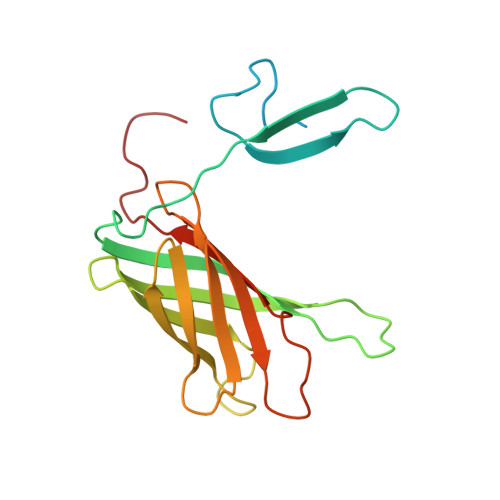
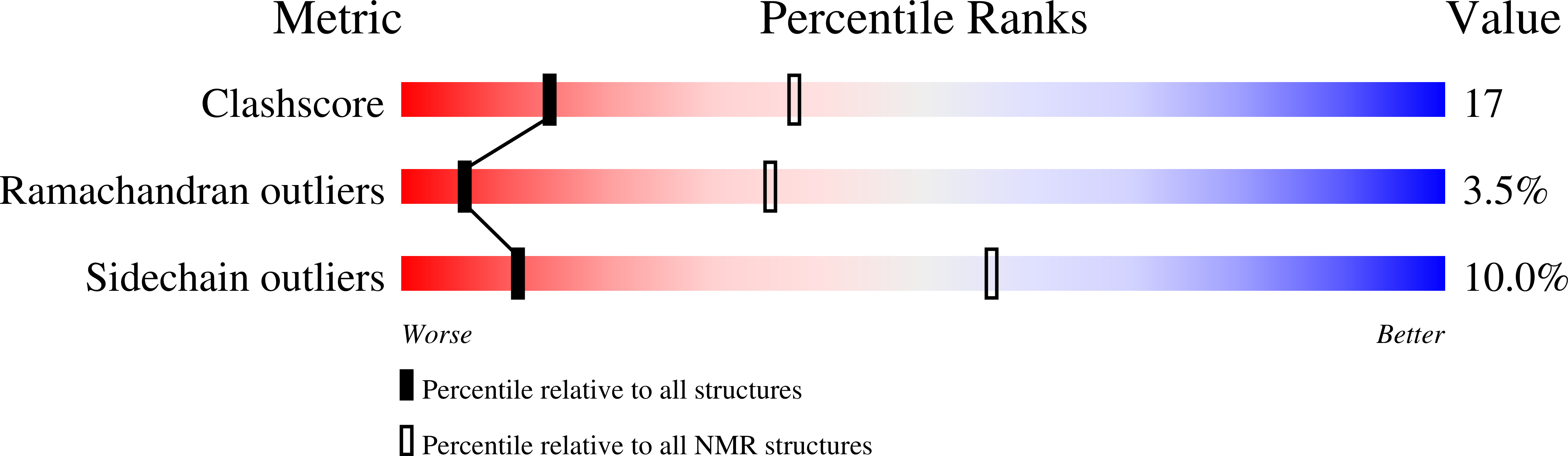Structure of the C-terminal Domain of Neisseria Heparin Binding Antigen (NHBA), One of the Main Antigens of a Novel Vaccine against Neisseria meningitidis.
Esposito, V., Musi, V., de Chiara, C., Veggi, D., Serruto, D., Scarselli, M., Kelly, G., Pizza, M., Pastore, A.(2011) J Biol Chem 286: 41767-41775
- PubMed: 21965688
- DOI: https://doi.org/10.1074/jbc.M111.289314
- Primary Citation of Related Structures:
2LFU - PubMed Abstract:
Neisseria heparin binding antigen (NHBA), also known as GNA2132 (genome-derived Neisseria antigen 2132), is a surface-exposed lipoprotein from Neisseria meningitidis that was originally identified by reverse vaccinology. It is one the three main antigens of a multicomponent vaccine against serogroup B meningitis (4CMenB), which has just completed phase III clinical trials in infants. In contrast to the other two main vaccine components, little is known about the origin of the immunogenicity of this antigen, and about its ability to induce a strong cross-bactericidal response in animals and humans. To characterize NHBA in terms of its structural/immunogenic properties, we have analyzed its sequence and identified a C-terminal region that is highly conserved in all strains. We demonstrate experimentally that this region is independently folded, and solved its three-dimensional structure by nuclear magnetic resonance. Notably, we need detergents to observe a single species in solution. The NHBA domain fold consists of an 8-strand β-barrel that closely resembles the C-terminal domains of N. meningitidis factor H-binding protein and transferrin-binding protein B. This common fold together with more subtle structural similarities suggest a common ancestor for these important antigens and a role of the β-barrel fold in inducing immunogenicity against N. meningitidis. Our data represent the first step toward understanding the relationship between structural, functional, and immunological properties of this important vaccine component.
Organizational Affiliation:
MRC National Institute for Medical Research, The Ridgeway, London NW71AA, United Kingdom.