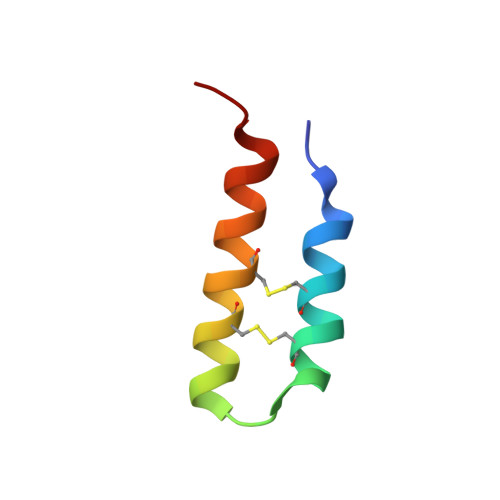
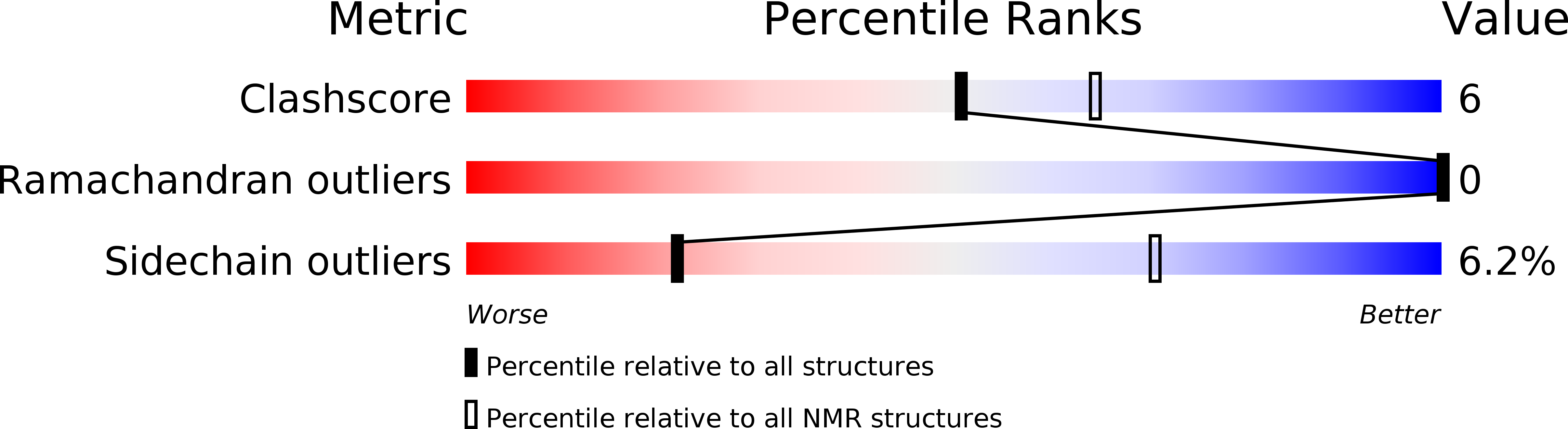Structural characterization and anti-HIV-1 activities of arginine/glutamate-rich polypeptide Luffin P1 from the seeds of sponge gourd (Luffa cylindrical).
Ng, Y.M., Yang, Y., Sze, K.H., Zhang, X., Zheng, Y.T., Shaw, P.C.(2010) J Struct Biol
- PubMed: 21195767
- DOI: https://doi.org/10.1016/j.jsb.2010.12.007
- Primary Citation of Related Structures:
2L37 - PubMed Abstract:
Luffin P1, the smallest ribosome-inactivating peptide from the seeds of Luffa cylindrica was found to have anti-HIV-1 activity in HIV-1 infected C8166 T-cell lines and be able to bind with HIV Rev Response Element. Nuclear magnetic resonance spectroscopy revealed that the Luffin P1 comprises a helix-loop-helix motif, with the two alpha helices tightly associated by two disulfide bonds. Based on our findings, we conclude that unlike the well-studied ribosome-inactivating proteins, which exert their action through N-glycosidase activities, Luffin P1 demonstrates a novel inactivation mechanism probably through the charge complementation with viral or cellular proteins. Our work also provides a new scaffold for the design of novel inhibitors from a simple helical motif.
Organizational Affiliation:
School of Life Sciences and Centre for Protein Science and Crystallography, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong, PR China.