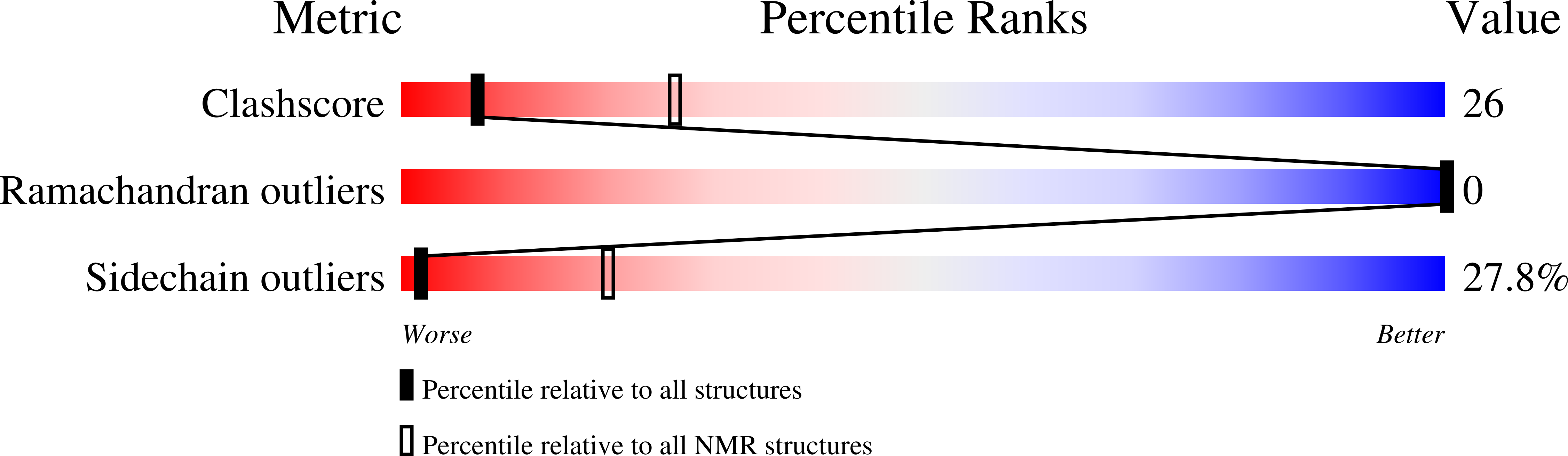Recombinant expression, synthesis, purification, and solution structure of arenicin
Ovchinnikova, T.V., Shenkarev, Z.O., Nadezhdin, K.D., Balandin, S.V., Zhmak, M.N., Kudelina, I.A., Finkina, E.I., Kokryakov, V.N., Arseniev, A.S.(2007) Biochem Biophys Res Commun 360: 156-162
- PubMed: 17585874
- DOI: https://doi.org/10.1016/j.bbrc.2007.06.029
- Primary Citation of Related Structures:
2JNI - PubMed Abstract:
Arenicins are 21-residue cationic antimicrobial peptides, isolated from marine polychaeta Arenicola marina. In order to determine a high-resolution three-dimensional structure of arenicin-2, the recombinant peptide was overexpressed as a fused form in Escherichia coli. Both arenicin isoforms were synthesized using the Fmoc-based solid-phase strategy. Recombinant and synthetic arenicins were purified, and their antimicrobial and spectroscopic properties were analyzed. NMR investigation shows that in water solution arenicin-2 displays a prolonged beta-hairpin, formed by two antiparallel beta-strands and stabilized by one disulfide and nine hydrogen bonds. A significant right-handed twist in the beta-sheet is deprived the peptide surface of amphipathicity. CD spectroscopic analysis indicates that arenicin-2 binds to the SDS and DPC micelles, and conformation of the peptide is significantly changed upon binding. Arenicin strongly binds to anionic lipid (POPE/POPG) vesicles in contrast with zwitterionic (POPC) ones. These results suggest that arenicins are membrane active peptides and point to possible mechanism of their selectivity toward bacterial cells.
Organizational Affiliation:
Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, Miklukho-Maklaya Str., 16/10, 117997 Moscow, Russia. ovch@ibch.ru