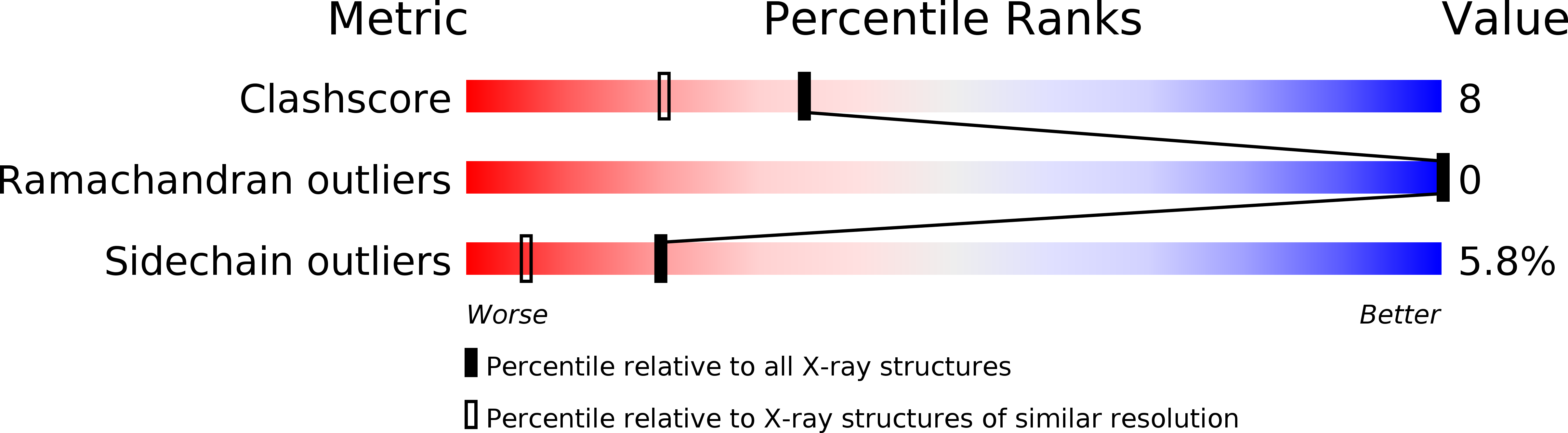Functional and Structural Aspects of Poplar Cytosolic and Plastidial Type a Methionine Sulfoxide Reductases
Rouhier, N., Kauffmann, B., Tete-Favier, F., Palladino, P., Gans, P., Branlant, G., Jacquot, J.P., Boschi-Muller, S.(2007) J Biol Chem 282: 3367
- PubMed: 17135266
- DOI: https://doi.org/10.1074/jbc.M605007200
- Primary Citation of Related Structures:
2J89 - PubMed Abstract:
The genome of Populus trichocarpa contains five methionine sulfoxide reductase A genes. Here, both cytosolic (cMsrA) and plastidial (pMsrA) poplar MsrAs were analyzed. The two recombinant enzymes are active in the reduction of methionine sulfoxide with either dithiothreitol or poplar thioredoxin as a reductant. In both enzymes, five cysteines, at positions 46, 81, 100, 196, and 202, are conserved. Biochemical and enzymatic analyses of the cysteine-mutated MsrAs support a catalytic mechanism involving three cysteines at positions 46, 196, and 202. Cys(46) is the catalytic cysteine, and the two C-terminal cysteines, Cys(196) and Cys(202), are implicated in the thioredoxin-dependent recycling mechanism. Inspection of the pMsrA x-ray three-dimensional structure, which has been determined in this study, strongly suggests that contrary to bacterial and Bos taurus MsrAs, which also contain three essential Cys, the last C-terminal Cys(202), but not Cys(196), is the first recycling cysteine that forms a disulfide bond with the catalytic Cys(46). Then Cys(202) forms a disulfide bond with the second recycling cysteine Cys(196) that is preferentially reduced by thioredoxin. In agreement with this assumption, Cys(202) is located closer to Cys(46) compared with Cys(196) and is included in a (202)CYG(204) signature specific for most plant MsrAs. The tyrosine residue corresponds to the one described to be involved in substrate binding in bacterial and B. taurus MsrAs. In these MsrAs, the tyrosine residue belongs to a similar signature as found in plant MsrAs but with the first C-terminal cysteine instead of the last C-terminal cysteine.
Organizational Affiliation:
UMR 1136 INRA-UHP, Nancy Universités, Interactions Arbres Microorganismes, IFR 110, Faculté des Sciences et Techniques, BP 239, 54506 Vandoeuvre Cedex, France. nrouhier@scbiol.uhp-anacy.fr