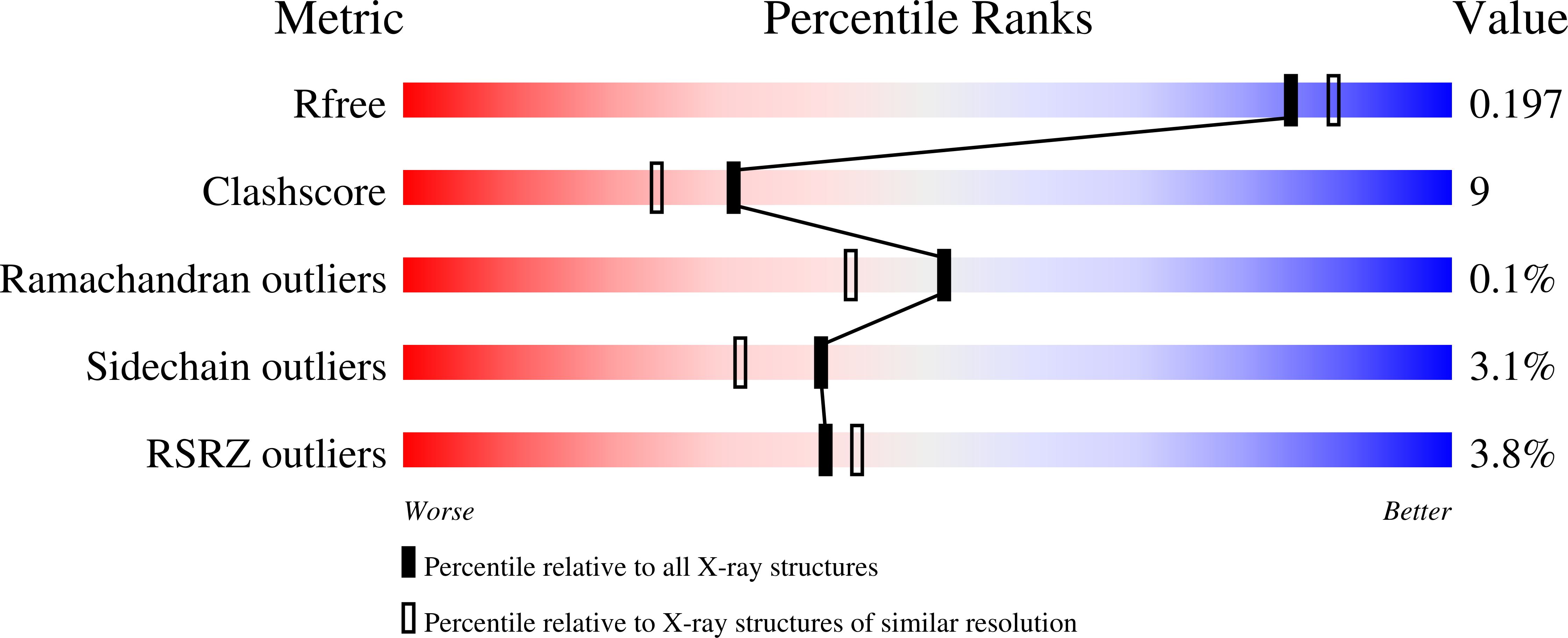
Structural basis for substrate binding and regioselective oxidation of monosaccharides at c3 by pyranose 2-oxidase.
Kujawa, M., Ebner, H., Leitner, C., Hallberg, B.M., Prongjit, M., Sucharitakul, J., Ludwig, R., Rudsander, U., Peterbauer, C., Chaiyen, P., Haltrich, D., Divne, C.(2006) J Biol Chem 281: 35104-35115
- PubMed: 16984920
- DOI: https://doi.org/10.1074/jbc.M604718200
- Primary Citation of Related Structures:
2IGK, 2IGM, 2IGN, 2IGO - PubMed Abstract:
Pyranose 2-oxidase (P2Ox) participates in fungal lignin degradation by producing the H2O2 needed for lignin-degrading peroxidases. The enzyme oxidizes cellulose- and hemicellulose-derived aldopyranoses at C2 preferentially, but also on C3, to the corresponding ketoaldoses. To investigate the structural determinants of catalysis, covalent flavinylation, substrate binding, and regioselectivity, wild-type and mutant P2Ox enzymes were produced and characterized biochemically and structurally. Removal of the histidyl-FAD linkage resulted in a catalytically competent enzyme containing tightly, but noncovalently bound FAD. This mutant (H167A) is characterized by a 5-fold lower kcat, and a 35-mV lower redox potential, although no significant structural changes were seen in its crystal structure. In previous structures of P2Ox, the substrate loop (residues 452-457) covering the active site has been either disordered or in a conformation incompatible with carbohydrate binding. We present here the crystal structure of H167A in complex with a slow substrate, 2-fluoro-2-deoxy-D-glucose. Based on the details of 2-fluoro-2-deoxy-D-glucose binding in position for oxidation at C3, we also outline a probable binding mode for D-glucose positioned for regioselective oxidation at C2. The tentative determinant for discriminating between the two binding modes is the position of the O6 hydroxyl group, which in the C2-oxidation mode can make favorable interactions with Asp452 in the substrate loop and, possibly, a nearby arginine residue (Arg472). We also substantiate our hypothesis with steady-state kinetics data for the alanine replacements of Asp452 and Arg472 as well as the double alanine 452/472 mutant.
Organizational Affiliation:
School of Biotechnology, Royal Institute of Technology, Albanova University Center, SE-106 91 Stockholm, Sweden.