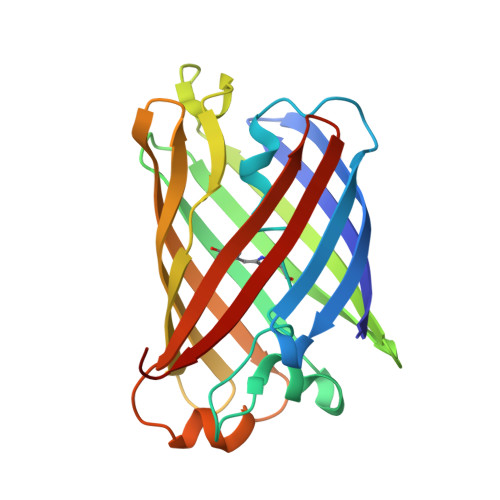
Structural basis for the fast maturation of Arthropoda green fluorescent protein.
Evdokimov, A.G., Pokross, M.E., Egorov, N.S., Zaraisky, A.G., Yampolsky, I.V., Merzlyak, E.M., Shkoporov, A.N., Sander, I., Lukyanov, K.A., Chudakov, D.M.(2006) EMBO Rep 7: 1006-1012
- PubMed: 16936637
- DOI: https://doi.org/10.1038/sj.embor.7400787
- Primary Citation of Related Structures:
2G6X, 2G6Y - PubMed Abstract:
Since the cloning of Aequorea victoria green fluorescent protein (GFP) in 1992, a family of known GFP-like proteins has been growing rapidly. Today, it includes more than a hundred proteins with different spectral characteristics cloned from Cnidaria species. For some of these proteins, crystal structures have been solved, showing diversity in chromophore modifications and conformational states. However, we are still far from a complete understanding of the origin, functions and evolution of the GFP family. Novel proteins of the family were recently cloned from evolutionarily distant marine Copepoda species, phylum Arthropoda, demonstrating an extremely rapid generation of fluorescent signal. Here, we have generated a non-aggregating mutant of Copepoda fluorescent protein and solved its high-resolution crystal structure. It was found that the protein beta-barrel contains a pore, leading to the chromophore. Using site-directed mutagenesis, we showed that this feature is critical for the fast maturation of the chromophore.
Organizational Affiliation:
X-ray crystallography, HCRC, Discovery, Procter&Gamble Pharmaceuticals, 8700 Mason-Montgomery Road, Mason, Ohio 45040, USA.