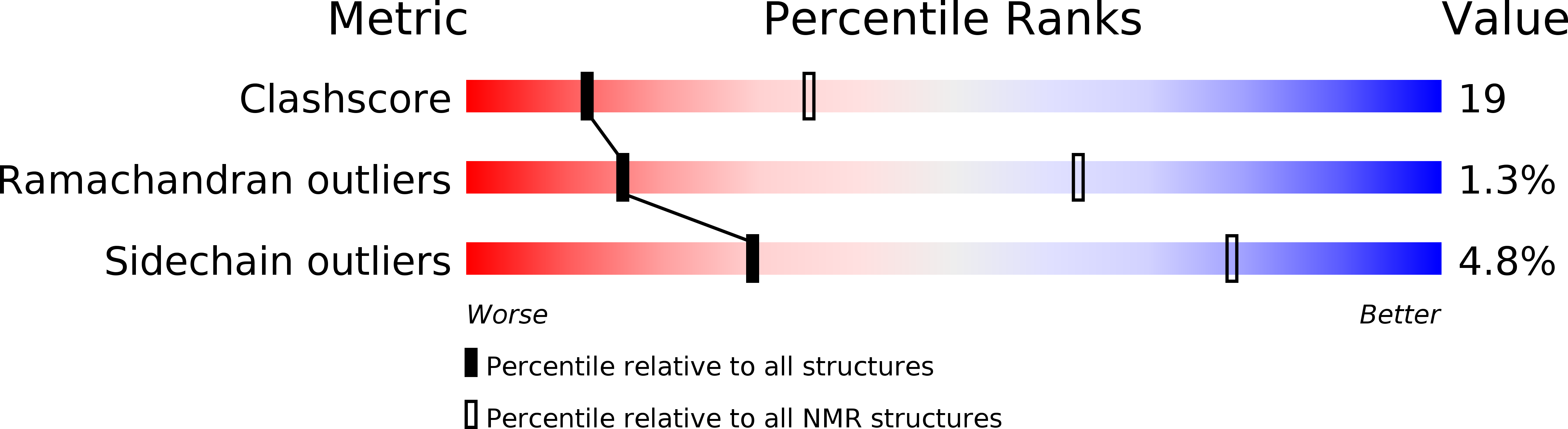Mutagenesis and Modeling of the Peroxiredoxin (Prx) Complex with the NMR Structure of ATP-Bound Human Sulfiredoxin Implicate Aspartate 187 of Prx I as the Catalytic Residue in ATP Hydrolysis
Lee, D.-Y., Park, S.J., Jeong, W., Sung, H.J., Oho, T., Wu, X., Rhee, S.G., Gruschus, J.M.(2006) Biochemistry 45: 15301-15309
- PubMed: 17176052
- DOI: https://doi.org/10.1021/bi061824h
- Primary Citation of Related Structures:
2B6F - PubMed Abstract:
The catalytic cysteine of certain members of the peroxiredoxin (Prx) family can be hyperoxidized to cysteinesulfinic acid during reduction of peroxides. Sulfiredoxin is responsible for the ATP-dependent reduction of cysteinesulfinic acid (SO2H) of hyperoxidized Prx. Here we report the NMR solution structure of human sulfiredoxin (hSrx), both with and without bound ATP, and we model the complex of ATP-bound hSrx with Prx. Binding ATP causes only small changes in the NMR structure of hSrx, and the bound ATP conformation is quite similar to that seen for the previously reported X-ray structure of the ADP-hSrx complex. Although hSrx binds ATP, it does not catalyze hydrolysis by itself and has no catalytic acid residue typical of most ATPase and kinase family proteins. For modeling the complex, the ATP-bound hSrx was docked to hyperoxidized Prx II using EMAP of CHARMM. In the model complex, Asn186 of Prx II (Asp187 of Prx I) is in contact with the hSrx-bound ATP beta- and gamma-phosphate groups. Asp187 of Prx I was mutated to alanine and asparagine, and binding and activity of the mutants with hSrx were compared to those of the wild type. For the D187N mutant, both binding and hydrolysis and reduction activities were comparable to those of the wild type, whereas for D187A, binding was unimpaired but ATP hydrolysis and reduction did not occur. The modeling and mutagenesis analyses strongly implicate Asp187 of Prx I as the catalytic residue responsible for ATP hydrolysis in the cysteinesulfinic acid reduction of Prx by hSrx.
Organizational Affiliation:
Laboratory of Cell Signaling, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland 20892-0301, USA.