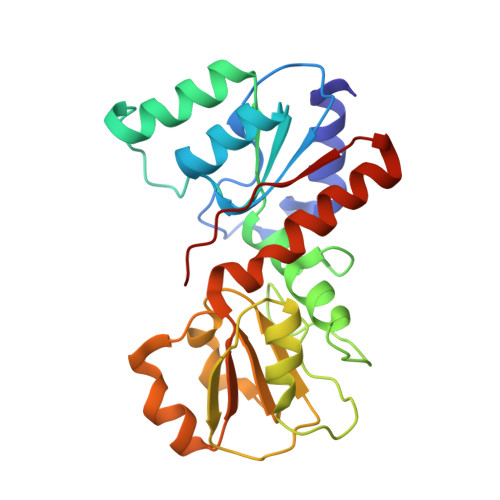
Crystal structure of the conserved hypothetical protein TTHA1606 from Thermus thermophilus HB8.
Tomoike, F., Wakamatsu, T., Nakagawa, N., Kuramitsu, S., Masui, R.(2009) Proteins 76: 244-248
- PubMed: 19322824
- DOI: https://doi.org/10.1002/prot.22397
- Primary Citation of Related Structures:
2YYB
Organizational Affiliation:
Graduate School of Frontier Biological Sciences, Osaka University, Osaka 565-0871, Japan.