Structural basis for sequence-spscific DNA recognition by an Arabidopsis WRKY transcription factor
Yamasaki, K., Kigawa, T., Watanabe, S., Inoue, M., Yamasaki, T., Seki, M., Shinozaki, K., Yokoyama, S.(2012) J Biol Chem
- PubMed: 22219184
- DOI: https://doi.org/10.1074/jbc.M111.279844
- Primary Citation of Related Structures:
2LEX - PubMed Abstract:
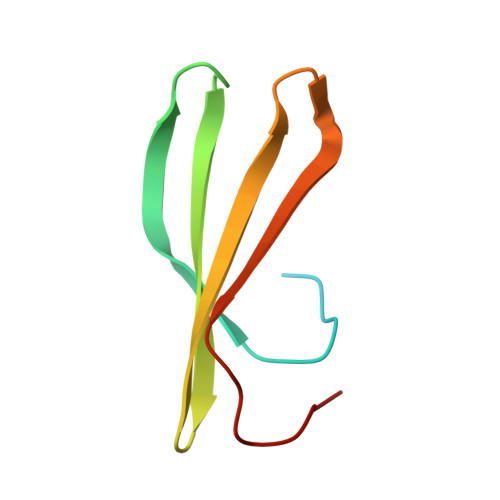
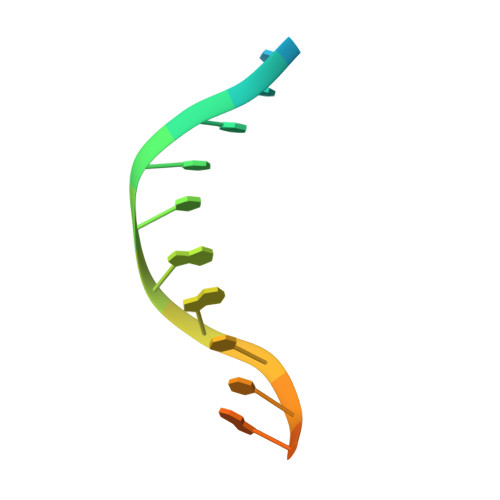
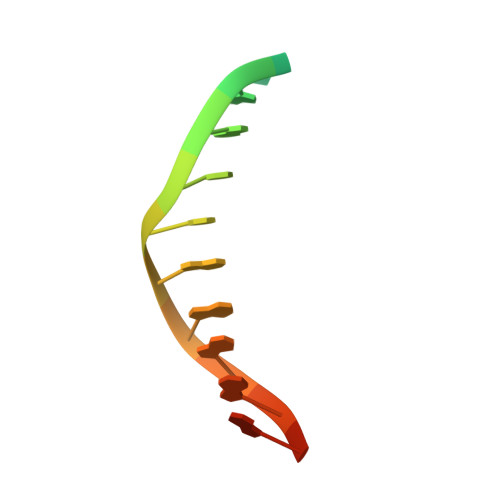
The WRKY family transcription factors regulate plant-specific reactions that are mostly related to biotic and abiotic stresses. They share the WRKY domain, which recognizes a DNA element (TTGAC(C/T)) termed the W-box, in target genes. Here, we determined the solution structure of the C-terminal WRKY domain of Arabidopsis WRKY4 in complex with the W-box DNA by NMR. A four-stranded β-sheet enters the major groove of DNA in an atypical mode termed the β-wedge, where the sheet is nearly perpendicular to the DNA helical axis. Residues in the conserved WRKYGQK motif contact DNA bases mainly through extensive apolar contacts with thymine methyl groups. The importance of these contacts was verified by substituting the relevant T bases with U and by surface plasmon resonance analyses of DNA binding.
Organizational Affiliation:
National Institute of Advanced Industrial Science and Technology (AIST), 1-1-1 Higashi, Tsukuba 305-8566, Japan. k-yamasaki@aist.go.jp