Structure of the Protease Production Regulatory Protein hpr from Bacillus subtilis.
Cuff, M.E., Skarina, T., Edwards, A., Savchenko, A., Joachimiak, A.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Protease production regulatory protein hpr | 207 | Bacillus subtilis | Mutation(s): 7 Gene Names: hpr, catA, scoC | 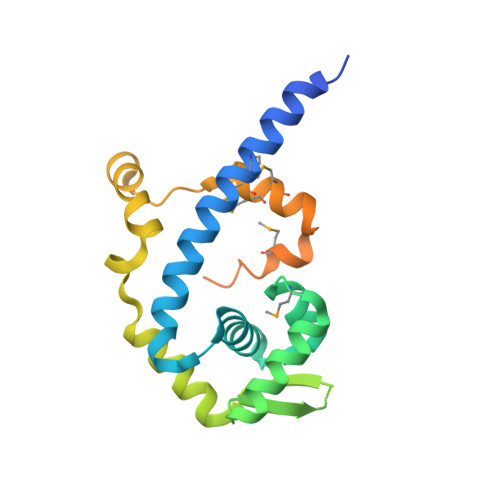 | |
UniProt | |||||
Find proteins for P11065 (Bacillus subtilis (strain 168)) Explore P11065 Go to UniProtKB: P11065 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P11065 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| P6G Query on P6G | P [auth B] | HEXAETHYLENE GLYCOL C12 H26 O7 IIRDTKBZINWQAW-UHFFFAOYSA-N |  | ||
| 1PE Query on 1PE | K [auth A], V [auth D] | PENTAETHYLENE GLYCOL C10 H22 O6 JLFNLZLINWHATN-UHFFFAOYSA-N |  | ||
| PGE Query on PGE | S [auth C] | TRIETHYLENE GLYCOL C6 H14 O4 ZIBGPFATKBEMQZ-UHFFFAOYSA-N |  | ||
| EDO Query on EDO | E [auth A] F [auth A] G [auth A] H [auth A] I [auth A] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A, B, C, D | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 52.272 | α = 90 |
| b = 79.909 | β = 100.18 |
| c = 131.239 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| d*TREK | data reduction |
| HKL-2000 | data scaling |
| CNS | phasing |
| autoSHARP | phasing |