Crystal structure of a putative trans-editing enzyme for prolyl tRNA synthetase
Murayama, K., Nakagawa, N., Ebihara, A., Kuramitsu, S., Shirouzu, M., Yokoyama, S.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| A PUTATIVE TRANS-EDITING ENZYME | 158 | Thermus thermophilus | Mutation(s): 0 | 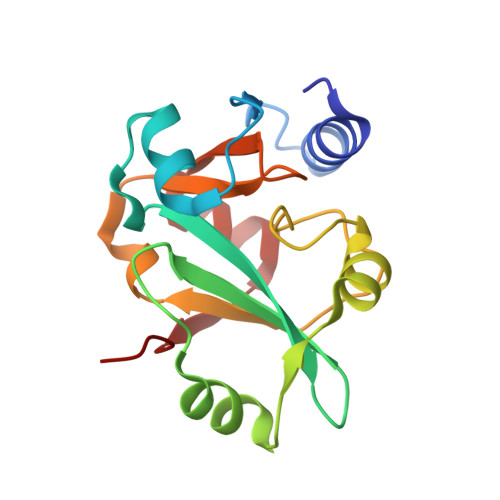 | |
UniProt | |||||
Find proteins for Q5SHN1 (Thermus thermophilus (strain ATCC 27634 / DSM 579 / HB8)) Explore Q5SHN1 Go to UniProtKB: Q5SHN1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q5SHN1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 61.576 | α = 90 |
| b = 61.245 | β = 110.77 |
| c = 84.186 | γ = 90 |
| Software Name | Purpose |
|---|---|
| CNS | refinement |
| HKL-2000 | data reduction |
| SCALEPACK | data scaling |
| MOLREP | phasing |