Molecular Recognition of Transcriptional Repressor Motifs by the Wd Domain of the Groucho/Tle Corepressor.
Jennings, B.H., Pickles, L.M., Wainwright, S.M., Roe, S.M., Pearl, L.H., Ish-Horowicz, D.(2006) Mol Cell 22: 645
- PubMed: 16762837
- DOI: https://doi.org/10.1016/j.molcel.2006.04.024
- Primary Citation of Related Structures:
2CE8, 2CE9 - PubMed Abstract:
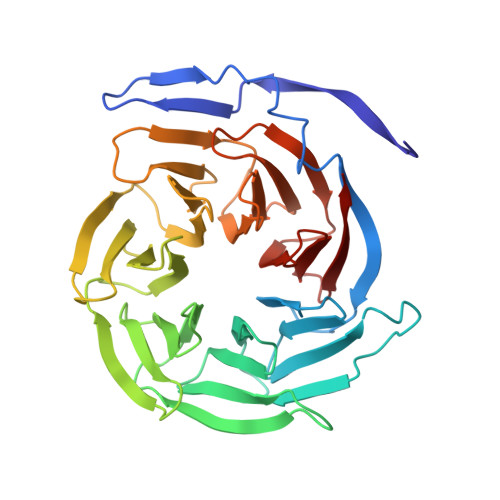
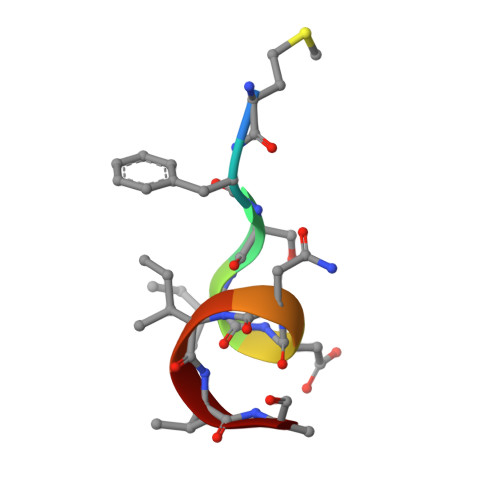
The Groucho (Gro)/TLE/Grg family of corepressors operates in many signaling pathways (including Notch and Wnt). Gro/TLE proteins recognize a wide range of transcriptional repressors by binding to divergent short peptide sequences, including a C-terminal WRPW/Y motif (Hairy/Hes/Runx) and internal eh1 motifs (FxIxxIL; Engrailed/Goosecoid/Pax/Nkx). Here, we identify several missense mutations in Drosophila Gro, which demonstrate peptide binding to the central pore of the WD (WD40) beta propeller domain in vitro and in vivo. We define these interactions at the molecular level with crystal structures of the WD domain of human TLE1 bound to either WRPW or eh1 peptides. The two distinct peptide motifs adopt markedly different bound conformations but occupy overlapping sites across the central pore of the beta propeller. Our structural and functional analysis explains the rigid conservation of the WRPW motif, the sequence flexibility of eh1 motifs, and other aspects of repressor recognition by Gro in vivo.
Organizational Affiliation:
Developmental Genetics Laboratory, Cancer Research UK, 44 Lincoln's Inn Fields, London WC2A 3PX, United Kingdom.