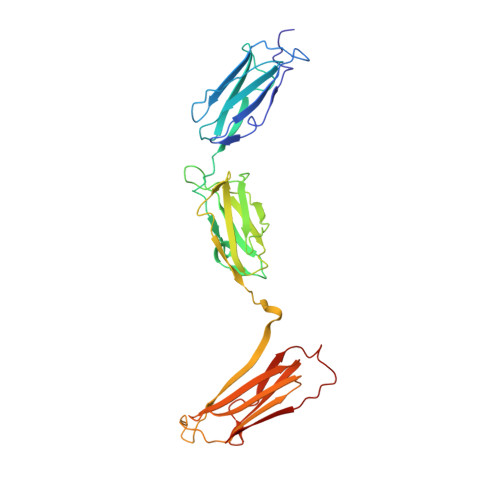
Molecular structure of the rod domain of dictyostelium filamin
Popowicz, G.M., Mueller, R., Noegel, A.A., Schleicher, M., Huber, R., Holak, T.A.(2004) J Mol Biol 342: 1637-1646
- PubMed: 15364587
- DOI: https://doi.org/10.1016/j.jmb.2004.08.017
- Primary Citation of Related Structures:
1WLH - PubMed Abstract:
Dictyostelium discoideum filamin (ddFLN) is a two-chain F-actin crosslinking protein with an N-terminal actin-binding domain and a rod domain constructed from six tandem repeats of a 100 residue motif that has an immunoglobulin (Ig) fold. We report the 2.8 A resolution crystal structure of a homodimer of rod repeats 4, 5 and 6. The two chains are arranged in an antiparallel fashion and form an elongated element, which is shortened, however, compared to a fully extended, linear configuration because the long axis of each Ig domain is arranged at an angle to the long axis of the rod. Same arrangement of repeats should also be present in the rod domain of human FLNa, much longer than Dictyostelium FLN, which forms an extended structure able to crosslink F-actin chains over distances of more than 1000 A.
Organizational Affiliation:
Max-Planck-Institut für Biochemie, 82152 Martinsried, Germany.