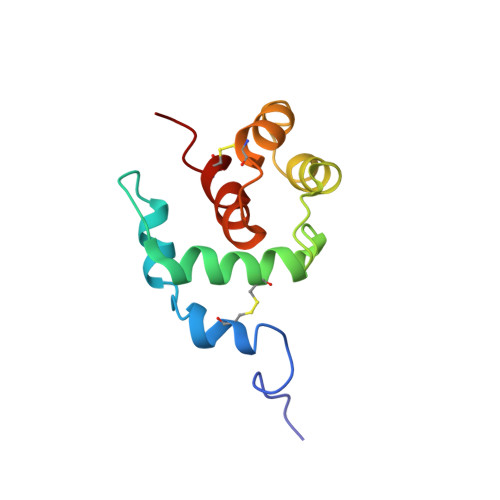
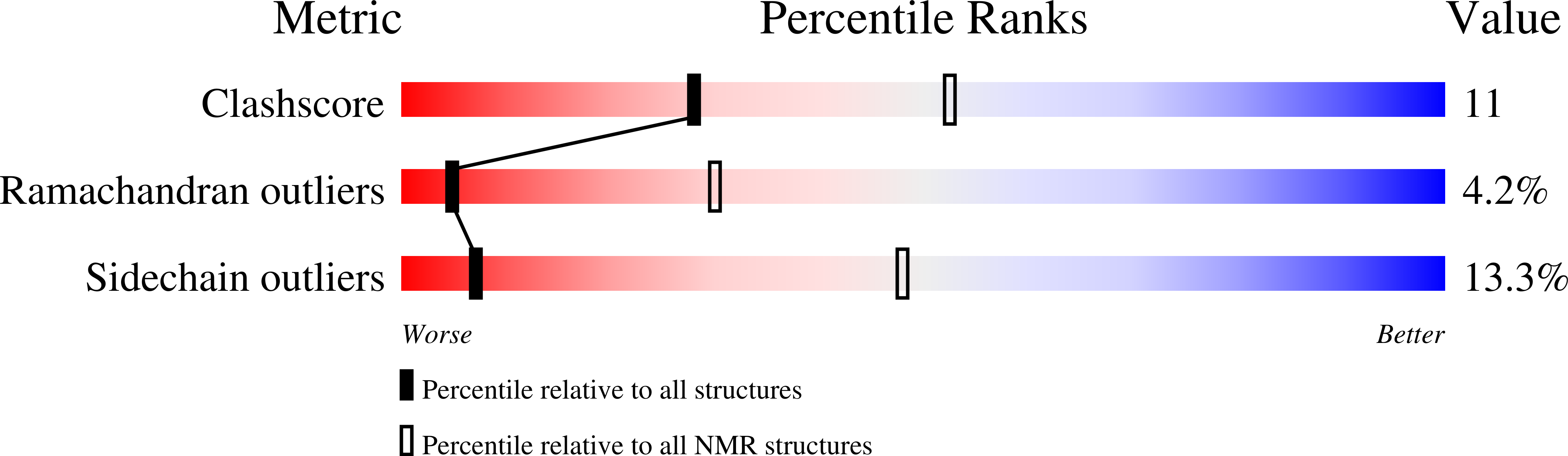A new class of hexahelical insect proteins revealed as putative carriers of small hydrophobic ligands.
Rothemund, S., Liou, Y.C., Davies, P.L., Krause, E., Sonnichsen, F.D.(1999) Structure 7: 1325-1332
- PubMed: 10574794
- DOI: https://doi.org/10.1016/s0969-2126(00)80022-2
- Primary Citation of Related Structures:
1C3Y, 1C3Z - PubMed Abstract:
THP12 is an abundant and extraordinarily hydrophilic hemolymph protein from the mealworm Tenebrio molitor and belongs to a group of small insect proteins with four highly conserved cysteine residues. Despite their sequence homology to odorant-binding proteins and pheromone-binding proteins, the function of these proteins is unclear. The first three-dimensional structure of THP12 has been determined by multidimensional NMR spectroscopy. The protein has a nonbundle helical structure consisting of six alpha helices. The arrangement of the alpha helices has a 'baseball glove' shape. In addition to the hydrophobic core, electrostatic interactions make contributions to the overall stability of the protein. NMR binding studies demonstrated the binding of small hydrophobic ligands to the single hydrophobic groove in THP12. Comparing the structure of THP12 with the predicted secondary structure of homologs reveals a common fold for this new class of insect proteins. A search with the program DALI revealed extensive similarity between the three-dimensional structure of THP12 and the N-terminal domain (residues 1-95) of recoverin, a member of the family of calcium-binding EF-hand proteins. Although the biological function of this new class of proteins is as yet undetermined, a general role as alpha-helical carrier proteins for small hydrophobic ligands, such as fatty acids or pheromones, is proposed on the basis of NMR-shift perturbation spectroscopy.
Organizational Affiliation:
Institute of Molecular Pharmacology, Berlin, 10315, Germany.