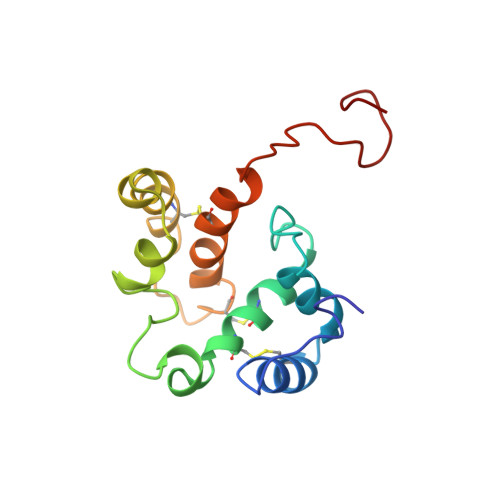
Structural Consequences of the pH-induced Conformational Switch in A.polyphemus Pheromone-binding Protein: Mechanisms of Ligand Release
Zubkov, S., Gronenborn, A.M., Byeon, I.J., Mohanty, S.(2005) J Mol Biol 354: 1081-1090
- PubMed: 16289114
- DOI: https://doi.org/10.1016/j.jmb.2005.10.015
- Primary Citation of Related Structures:
1TWO - PubMed Abstract:
Olfaction in moths is one of the most impressive examples of chemical communication found in nature for its exquisite sensitivity and selectivity. Pheromone-binding proteins (PBPs), present in the antennae of male moth and other insect species, bind the hydrophobic pheromone molecules and transport them to the G protein-coupled olfactory receptor proteins. The targeted delivery of these non-polar ligands to membrane-bound receptors involves ligand release on or near the target cell membranes, the molecular details of which are still not well understood. The PBP from the giant silk moth Antheraea polyphemus (ApolPBP) binds acetate pheromone only at pH above 6.0, and its structure at pH 6.3 has been determined previously. Here we report the solution NMR structure of ApolPBP at the acidic pH 5.2. Comparison of the present structure to that at neutral pH reveals the details of the pH-induced conformational changes and provides mechanistic clues for ligand release at acidic pH. The ApolPBP pH-induced structural change is quite different from that observed for alcohol binding Bombyx mori PBP (BmorPBP), where the C-terminal segment folds into a helix and occupies the ligand binding cavity. We observe a reorientation of helices alpha1, alpha3, and alpha4 at acidic pH caused by protonation of His69, His70 and His95 in the interior. This provides the driving force behind the opening of the ligand binding cavity and the release of the pheromone molecule from its carrier protein near the membrane.
Organizational Affiliation:
Department of Biochemistry and Cell Biology, Stony Brook University, Stony Brook, NY 11794-5215, USA.