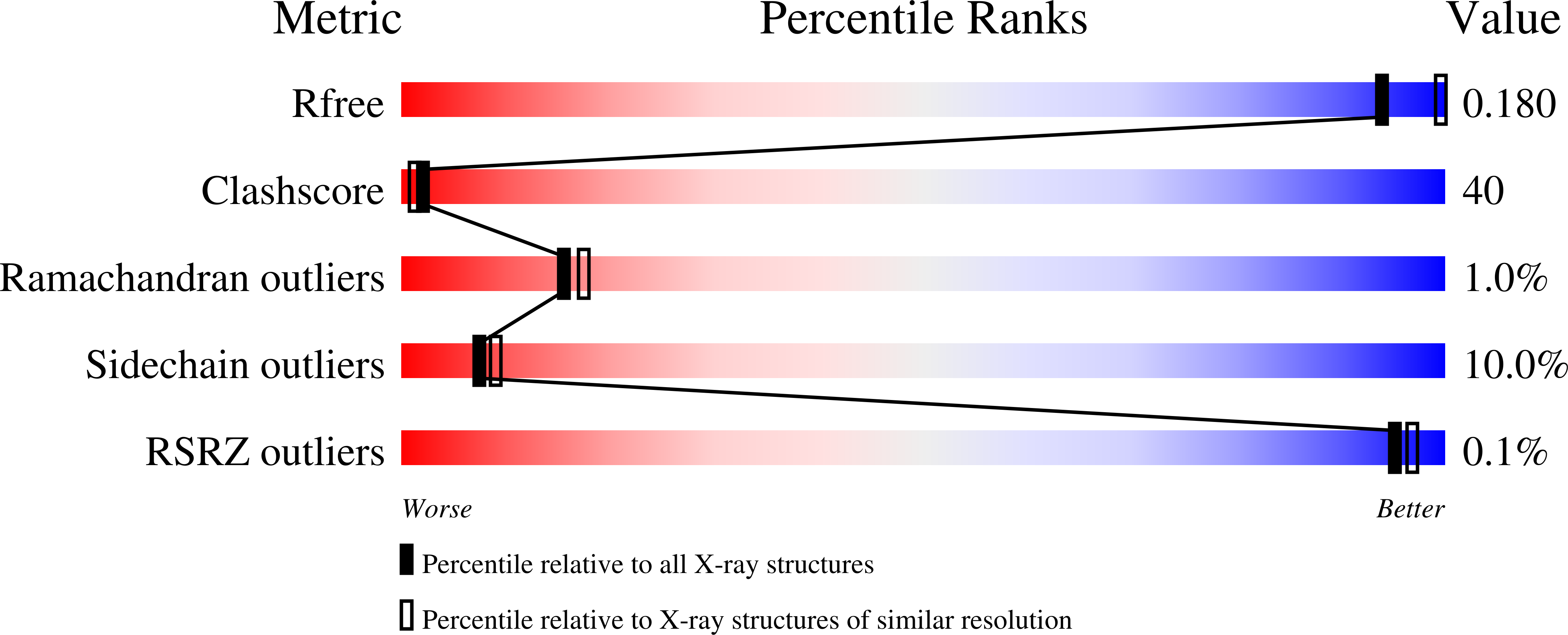
Evolution of Enzymatic Activity in the Enolase Superfamily: Structural Studies of the Promiscuous o-Succinylbenzoate Synthase from Amycolatopsis
Thoden, J.B., Taylor-Ringia, E.A., Garrett, J.B., Gerlt, J.A., Holden, H.M., Rayment, I.(2004) Biochemistry 43: 5716-5727
- PubMed: 15134446
- DOI: https://doi.org/10.1021/bi0497897
- Primary Citation of Related Structures:
1SJA, 1SJB, 1SJC, 1SJD - PubMed Abstract:
Divergent evolution of enzyme function is commonly explained by a gene duplication event followed by mutational changes that allow the protein encoded by the copy to acquire a new function. An alternate hypothesis is that this process is facilitated when the progenitor enzyme acquires a second function while maintaining the original activity. This phenomenon has been suggested to occur in the o-succinylbenzoate synthase (OSBS) from a species of Amycolatopsis that catalyzes not only the physiological syn-dehydration reaction of 2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate but also an accidental racemization of N-acylamino acids [Palmer, D. R., Garrett, J. B., Sharma, V., Meganathan, R., Babbitt, P. C., and Gerlt, J. A. (1999) Biochemistry 38, 4252-4258]. To understand the molecular basis of this promiscuity, three-dimensional structures of liganded complexes of this enzyme have been determined, including the product of the OSBS reaction and three N-acylamino acid substrates for the N-acylamino acid racemase (NAAAR) reaction, N-acetylmethionine, N-succinylmethionine, and N-succinylphenylglycine, to 2.2, 2.3, 2.1, and 1.9 A resolution, respectively. These structures show how the active-site cavity can accommodate both the hydrophobic substrate for the OSBS reaction and the substrates for the accidental NAAAR reaction. As expected, the N-acylamino acid is sandwiched between lysines 163 and 263, which function as the catalytic bases for the abstraction of the alpha-proton in the (R)- and (S)-racemization reactions, respectively [Taylor Ringia, E. A., Garrett, J. B, Thoden, J. B., Holden, H. M., Rayment, I., and Gerlt, J. A. (2004) Biochemistry 42, 224-229]. Importantly, the protein forms specific favorable interactions with the hydrophobic amino acid side chain, alpha-carbon, carboxylate, and the polar components of the N-acyl linkage. Accommodation of the components of the N-acyl linkage appears to be the reason that this enzyme is capable of a racemization reaction on these substrates, whereas the orthologous OSBS from Escherichia coli lacks this functionality.
Organizational Affiliation:
Department of Chemistry, University of Illinois, Urbana, Illinois 61801, USA.