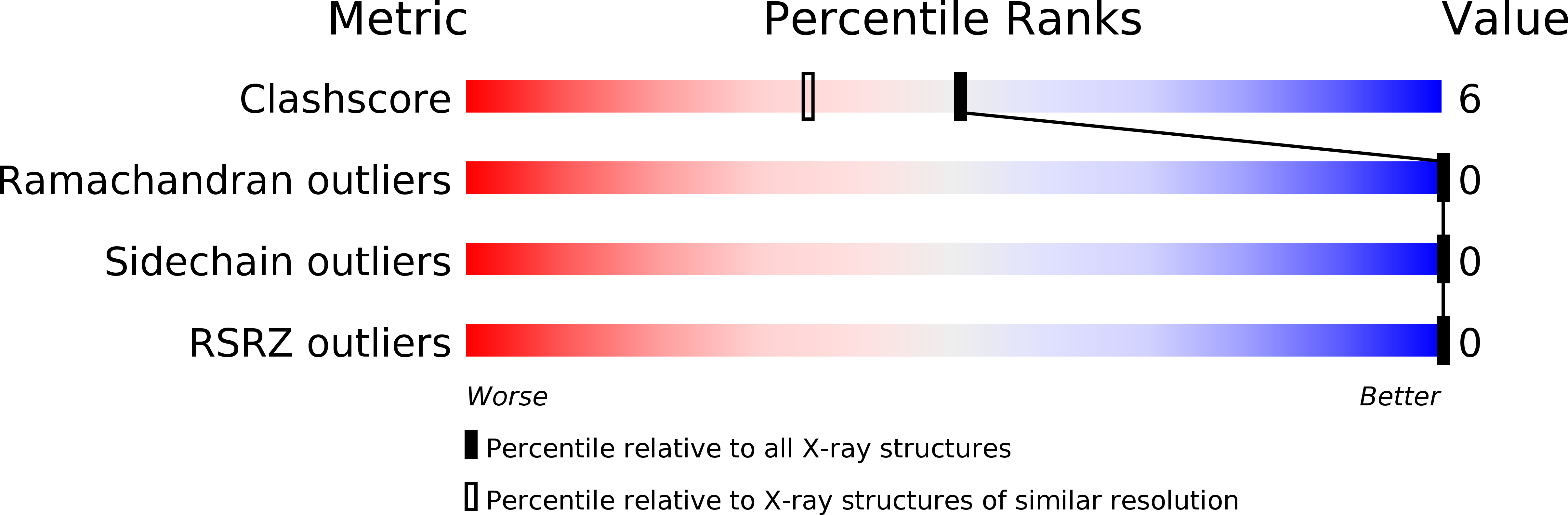
Crystal Structure of Vancomycin.
Schafer, M., Schneider, T.R., Sheldrick, G.M.(1996) Structure 4: 1509
- PubMed: 8994975
- DOI: https://doi.org/10.1016/s0969-2126(96)00156-6
- Primary Citation of Related Structures:
1SHO - PubMed Abstract:
Vancomycin and other related glycopeptide antibiotics are clinically very important because they often represent the last line of defence against bacteria that have developed resistance to antibiotics. Vancomycin is believed to act by binding nascent cell wall mucopeptides terminating in the sequence D-Ala-D-Ala, weakening the resulting cell wall. Extensive NMR and other studies have shown that the formation of asymmetric antibiotic dimers is important in peptide binding. Despite intensive efforts the crystal structure of vancomycin has been extremely difficult to obtain, partly because high-resolution data were unavailable, and partly because the structure was too large to be solved by conventional "direct methods'. Using low-temperature synchrotron X-ray data combined with new ab initio techniques for solving the crystallographic phase problem, we have succeeded in determining the crystal structure of vancomycin at atomic resolution. The structure provides much detailed information that should prove invaluable in modelling and mechanistic studies. Our structure confirms that vancomycin exists as an asymmetric dimer. The dimer conformation allows the docking of two D-Ala-D-Ala peptides in opposite directions; these presumably would be attached to different glycopeptide strands. In the crystal, one of the binding pockets is occupied by an acetate ion that mimics the C terminus of the nascent cell wall peptide; the other is closed by the asparagine sidechain, which occupies the place of a ligand. The occupied binding pocket exhibits high flexibility but the closed binding pocket is relatively rigid. We propose that the asparagine sidechain may hold the binding pocket in a suitable conformation for peptide docking, swinging out of the way when the peptide enters the binding pocket.
Organizational Affiliation:
Institut für Anorganische Chemie, Universität Göttingen, Germany.