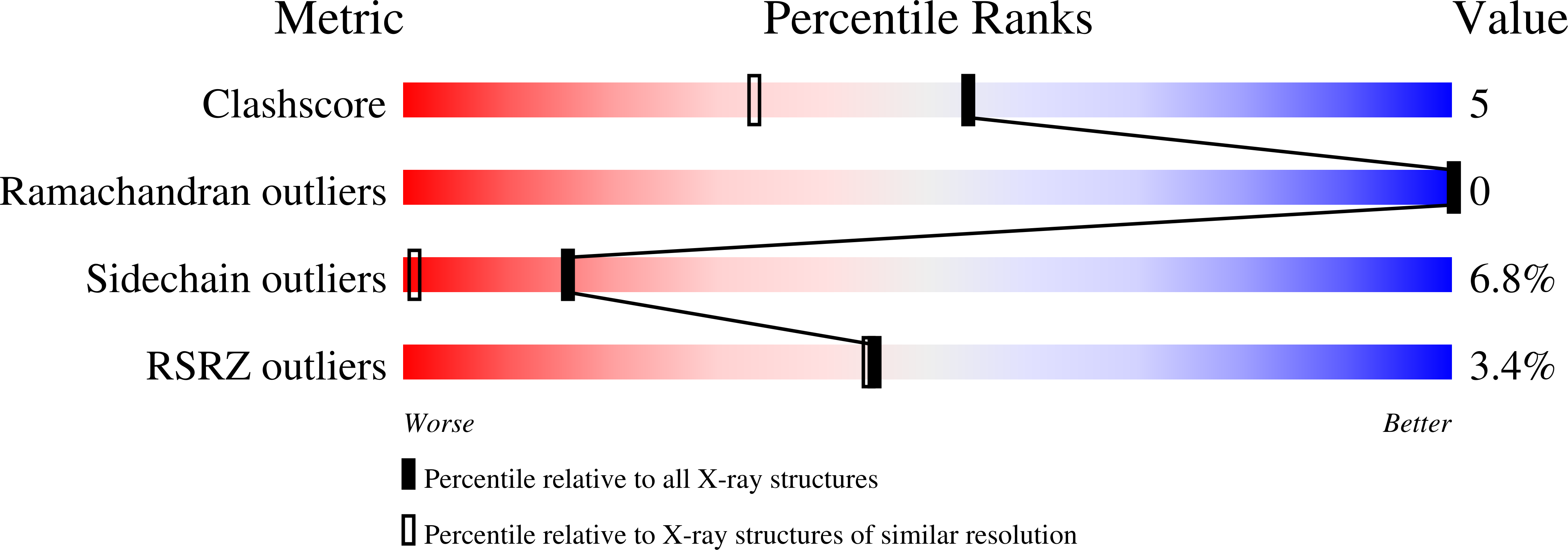
High Resolution Structure of Bovine Pancreatic Trypsin Inhibitor with Altered Binding Loop Sequence
Czapinska, H., Otlewski, J., Krzywda, S., Sheldrick, G.M., Jaskolski, M.(1999) J Mol Biol 295: 1237
- PubMed: 10653700
- DOI: https://doi.org/10.1006/jmbi.1999.3445
- Primary Citation of Related Structures:
1QLQ - PubMed Abstract:
A mutant of bovine pancreatic trypsin inhibitor (BPTI) has been constructed and expressed in Escherichia coli in order to probe the kinetic and structural consequences of truncating the binding loop residues to alanine. In addition to two such mutations (Thr11Ala and Pro13Ala), it has a conservative Lys15Arg substitution at position P(1) and an unrelated Met52Leu change. In spite of the binding loop modification, the affinity for trypsin is only 30 times lower than that of the wild-type protein. At pH 7.5 the protein can be crystallized on the time-scale of hours, yielding very stable crystals of a new (tetragonal) form of BPTI. Conventional source X-ray data collected to 1.4 A at room temperature allowed anisotropic structure refinement characterized by R=0.1048. The structure reveals all 58 residues, including the complete C terminus, which is in a salt-bridge contact with the N terminus. The Cys14-Cys38 disulfide bridge is observed in two distinct chiralities. This bridge, together with an internal water molecule, contributes to the stabilization of the binding loop. The Ala mutations have only an insignificant and localized effect on the binding loop, which retains its wild-type conformation (maximum deviation of loop C(alpha) atoms of 0.7 A at Ala13). Four (instead of the typical three) additional water molecules are buried in an internal cleft and connected to the surface via a sulfate anion. Three more SO(4)(2-) anions are seen in the electron density, one of them located on a 2-fold axis. It participates in the formation of a dimeric structure between symmetry-related BPTI molecules, in which electrostatic and hydrogen bonding interactions resulting from the mutated Lys15Arg substitution are of central importance. This dimeric interaction involves direct recognition loop-recognition loop contacts, part of which are hydrophobic interactions of the patches created by the alanine mutations. Another 2-fold symmetric interaction between the BPTI molecules involves the formation of an antiparallel intermolecular beta-sheet that, together with the adjacent intramolecular beta-hairpin loops, creates a four-stranded structure.
Organizational Affiliation:
Department of Protein Engineering, Institute of Biochemistry and Molecular Biology, University of Wroclaw, Tamka 2, Wroclaw, 50-137, Poland.