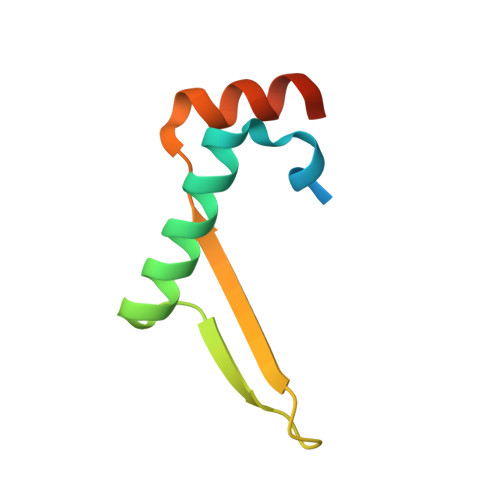
Structure of the nucleocapsid protein of porcine reproductive and respiratory syndrome virus.
Doan, D.N.P., Dokland, T.(2003) Structure 11: 1445-1451
- PubMed: 14604534
- DOI: https://doi.org/10.1016/j.str.2003.09.018
- Primary Citation of Related Structures:
1P65 - PubMed Abstract:
Porcine reproductive and respiratory syndrome virus (PRRSV) is an enveloped RNA virus of the Arteriviridae family, genomically related to the coronaviruses. PRRSV is the causative agent of both severe and persistent respiratory disease and reproductive failure in pigs worldwide. The PRRSV virion contains a core made of the 123 amino acid nucleocapsid (N) protein, a product of the ORF7 gene. We have determined the crystal structure of the capsid-forming domain of N. The structure was solved to 2.6 A resolution by SAD methods using the anomalous signal from sulfur. The N protein exists in the crystal as a tight dimer forming a four-stranded beta sheet floor superposed by two long alpha helices and flanked by two N- and two C-terminal alpha helices. The structure of N represents a new class of viral capsid-forming domains, distinctly different from those of other known enveloped viruses, but reminiscent of the coat protein of bacteriophage MS2.
Organizational Affiliation:
Institute of Molecular and Cell Biology, 117609 Singapore, Singapore.