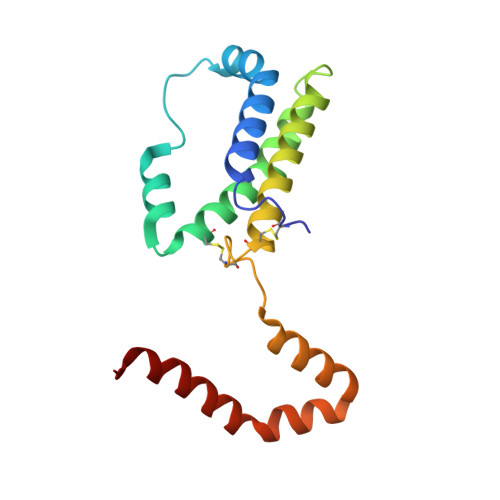
Crystal structure of interleukin-10 reveals the functional dimer with an unexpected topological similarity to interferon gamma.
Zdanov, A., Schalk-Hihi, C., Gustchina, A., Tsang, M., Weatherbee, J., Wlodawer, A.(1995) Structure 3: 591-601
- PubMed: 8590020
- DOI: https://doi.org/10.1016/s0969-2126(01)00193-9
- Primary Citation of Related Structures:
1ILK - PubMed Abstract:
Interleukin (IL)-10 is a cytokine that inhibits production of other regulatory factors, including interferon gamma (IFN-gamma) and IL-2. A dimer of IL-10 is present in solution and is presumed to participate in receptor binding, but the nature of the dimer has not been previously reported. An atomic model is necessary to interpret biological activity of IL-10 and to design mutants with agonistic or antagonistic properties.
Organizational Affiliation:
Macromolecular Structure Laboratory, NCI-Frederick Cancer Research and Development Center, Maryland 21702, USA.