Solution Structure of the Ldl Receptor Egf-Ab Pair: A Paradigm for the Assembly of Tandem Calcium Binding Egf Domains
Saha, S., Boyd, J., Werner, J.M., Knott, V., Handford, P.A., Campbell, I.D., Downing, A.K.(2001) Structure 9: 451
- PubMed: 11435110
- DOI: https://doi.org/10.1016/s0969-2126(01)00606-2
- Primary Citation of Related Structures:
1HJ7 - PubMed Abstract:
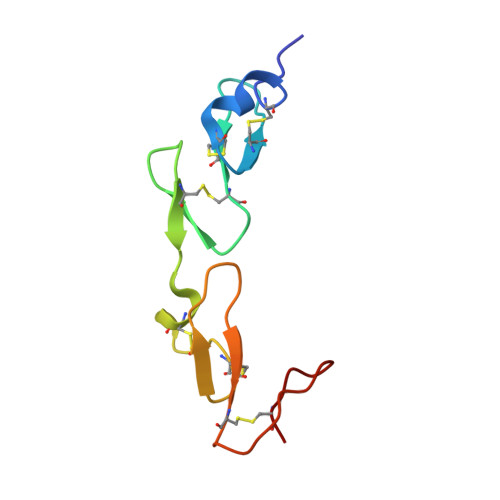
From the observed structure and sequence of a pair of calcium binding (cb) epidermal growth factor-like (EGF) domains from human fibrillin-1, we proposed that many tandem cbEGF domains adopt a conserved relative conformation. The low-density lipoprotein receptor (LDLR), which is functionally unrelated to fibrillin-1, contains a single pair of EGF domains that was chosen for study in the validation of this hypothesis. The LDLR is the protein that is defective in familial hypercholesterolaemia, a common genetic disorder that predisposes individuals to cardiovascular complications and premature death. Here, we present the solution structure of the first two EGF domains from the LDL receptor, determined using conventional NMR restraints and residual dipolar couplings. The cbEGF domains have an elongated, rod-like arrangement, as predicted. The new structure allows a detailed assessment of the consequences of mutations associated with familial hypercholesterolaemia to be made. The validation of the conserved arrangement of EGF domains in functionally distinct proteins has important implications for structural genomics, since multiple tandem cbEGF pairs have been identified in many essential proteins that are implicated in human disease. Our results provide the means to use homology modeling to probe structure-function relationships in this diverse family of proteins and may hold the potential for the design of novel diagnostics and therapies in the future.
Organizational Affiliation:
Division of Structural Biology, Department of Biochemistry, University of Oxford, South Parks Road, OX1 3QU, Oxford, United Kingdom.