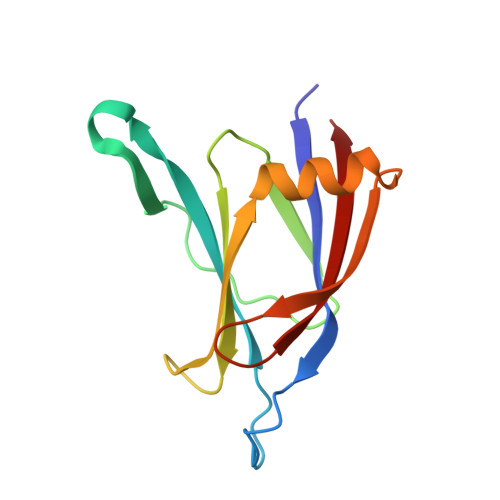
Crystal structure of the C2 domain from protein kinase C-delta.
Pappa, H., Murray-Rust, J., Dekker, L.V., Parker, P.J., McDonald, N.Q.(1998) Structure 6: 885-894
- PubMed: 9687370
- DOI: https://doi.org/10.1016/s0969-2126(98)00090-2
- Primary Citation of Related Structures:
1BDY - PubMed Abstract:
The protein kinase C (PKC) family of lipid-dependent serine/theonine kinases plays a central role in many intracellular eukaryotic signalling events. Members of the novel (delta, epsilon, eta, theta) subclass of PKC isotypes lack the Ca2+ dependence of the conventional PKC isotypes and have an N-terminal C2 domain, originally defined as V0 (variable domain zero). Biochemical data suggest that this domain serves to translocate novel PKC family members to the plasma membrane and may influence binding of PKC activators.
Organizational Affiliation:
Structural Biology, Imperial Cancer Research Fund, London, UK.