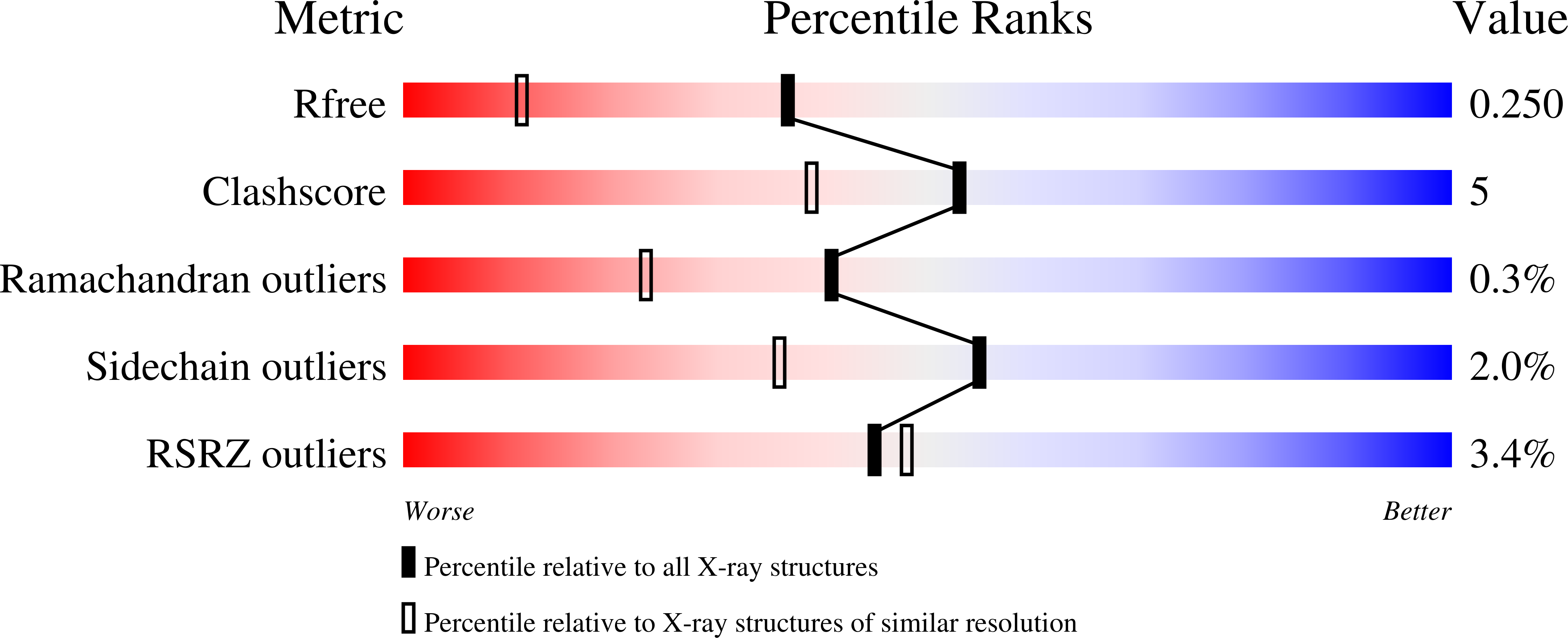
Systematic mutational analysis of human neutrophil alpha-defensin HNP4.
Hu, H., Di, B., Tolbert, W.D., Gohain, N., Yuan, W., Gao, P., Ma, B., He, Q., Pazgier, M., Zhao, L., Lu, W.(2019) Biochim Biophys Acta Biomembr 1861: 835-844
- PubMed: 30658057
- DOI: https://doi.org/10.1016/j.bbamem.2019.01.007
- Primary Citation of Related Structures:
6DMM, 6DMQ - PubMed Abstract:
Defensins are a family of cationic antimicrobial peptides of innate immunity with immunomodulatory properties. The prototypic human α-defensins, also known as human neutrophil peptides 1-3 or HNP1-3, are extensively studied for their structure, function and mechanisms of action, yet little is known about HNP4 - the much less abundant "distant cousin" of HNP1-3. Here we report a systematic mutational analysis of HNP4 with respect to its antibacterial activity against E. coli and S. aureus, inhibitory activity against anthrax lethal factor (LF), and binding activity for LF and HIV-1 gp120. Except for nine conserved and structurally important residues (6xCys, 1xArg, 1xGlu and 1xGly), the remaining 24 residues of HNP4 were each individually mutated to Ala. The crystal structures of G23A-HNP4 and T27A-HNP4 were determined, both exhibiting a disulfide-stabilized canonical α-defensin dimer identical to wild-type HNP4. Unlike HNP1-3, HNP4 preferentially killed the Gram-negative bacterium, a property largely attributable to three clustered cationic residues Arg10, Arg11 and Arg15. The cationic cluster was also important for HNP4 killing of S. aureus, inhibition of LF and binding to LF and gp120. However, F26A, while functionally inconsequential for E. coli killing, was far more deleterious than any other mutations. Similarly, N-methylation of Leu20 to destabilize the HNP4 dimer had little effect on E. coli killing, but significantly reduced the ability of HNP4 to kill S. aureus, inhibit LF, and bind to LF and gp120. Our findings unveil the molecular determinants of HNP4 function, completing the atlas of structure and function relationships for all human neutrophil α-defensins.
Organizational Affiliation:
Key Laboratory of Fermentation Engineering, Ministry of Education, College of Bioengineering, Hubei University of Technology, Wuhan, China; Institute of Human Virology, Department of Biochemistry and Molecular Biology, University of Maryland School of Medicine, Baltimore, USA.