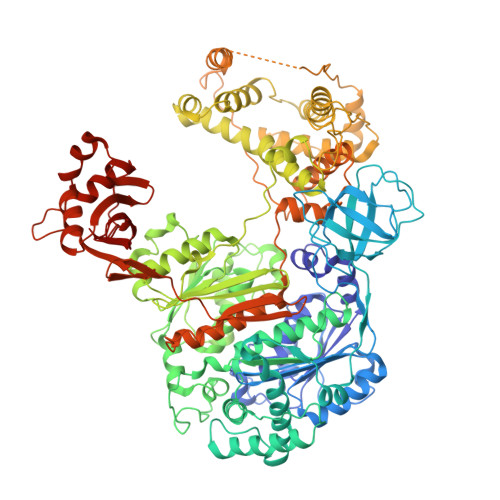
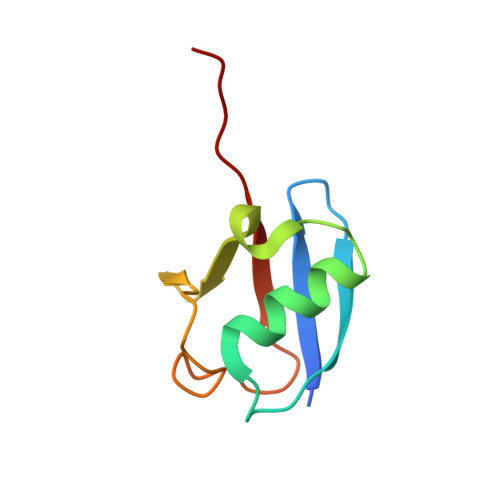
Crystal structure of a human ubiquitin E1-ubiquitin complex reveals conserved functional elements essential for activity.
Lv, Z., Williams, K.M., Yuan, L., Atkison, J.H., Olsen, S.K.(2018) J Biol Chem 293: 18337-18352
- PubMed: 30279270
- DOI: https://doi.org/10.1074/jbc.RA118.003975
- Primary Citation of Related Structures:
6DC6 - PubMed Abstract:
Ubiquitin (Ub) signaling plays a key regulatory role in nearly every aspect of eukaryotic biology and is initiated by E1 enzymes that activate and transfer Ub to E2 Ub-conjugating enzymes. Despite Ub E1's fundamental importance to the cell and its attractiveness as a target for therapeutic intervention in cancer and other diseases, its only available structural information is derived from yeast orthologs of human ubiquitin-like modifier-activating enzyme 1 (hUBA1). To illuminate structural differences between yeast and hUBA1 structures that might be exploited for the development of small-molecule therapeutics, we determined the first crystal structure of a hUBA1-Ub complex. Using structural analysis, molecular modeling, and biochemical analysis, we demonstrate that hUBA1 shares a conserved overall structure and mechanism with previously characterized yeast orthologs, but displays subtle structural differences, particularly within the active site. Computational analysis revealed four potential ligand-binding hot spots on the surface of hUBA1 that might serve as targets to inhibit hUBA1 at the level of Ub activation or E2 recruitment or that might potentially be used in approaches such as protein-targeting chimeric molecules. Taken together, our work enhances our understanding of the hUBA1 mechanism, provides an improved framework for the development of small-molecule inhibitors of UBA1, and serves as a stepping stone for structural studies that involve the enzymes of the human Ub system at the level of both E1 and E2.
Organizational Affiliation:
From the Department of Biochemistry and Molecular Biology and Hollings Cancer Center, Medical University of South Carolina, Charleston, South Carolina 29425.