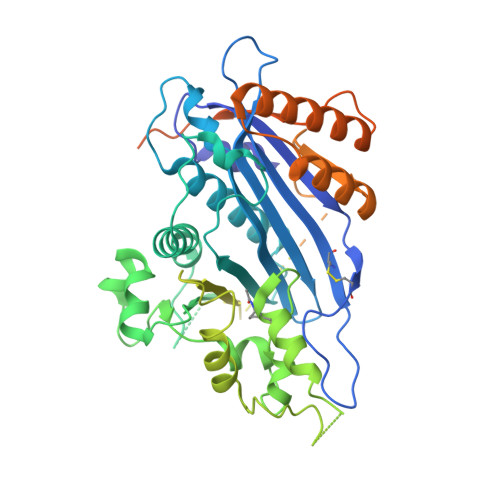
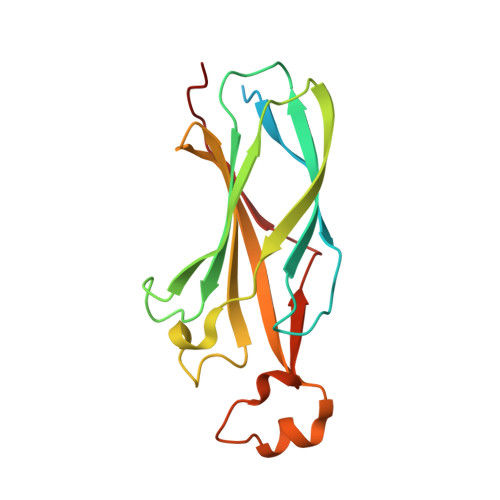
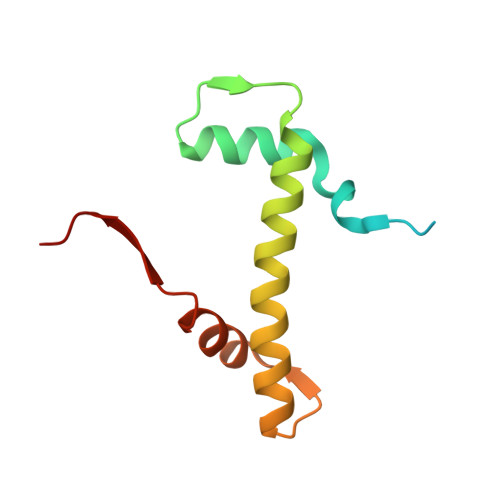
Multisite Substrate Recognition in Asf1-Dependent Acetylation of Histone H3 K56 by Rtt109.
Zhang, L., Serra-Cardona, A., Zhou, H., Wang, M., Yang, N., Zhang, Z., Xu, R.M.(2018) Cell 174: 818-830.e11
- PubMed: 30057113
- DOI: https://doi.org/10.1016/j.cell.2018.07.005
- Primary Citation of Related Structures:
5ZB9, 5ZBA, 5ZBB - PubMed Abstract:
Rtt109 is a unique histone acetyltransferase acetylating histone H3 lysine 56 (H3K56), a modification critical for DNA replication-coupled nucleosome assembly and genome stability. In cells, histone chaperone Asf1 is essential for H3K56 acetylation, yet the mechanisms for H3K56 specificity and Asf1 requirement remain unknown. We have determined the crystal structure of the Rtt109-Asf1-H3-H4 complex and found that unwinding of histone H3 α N , where K56 is normally located, and stabilization of the very C-terminal β strand of histone H4 by Asf1 are prerequisites for H3K56 acetylation. Unexpectedly, an interaction between Rtt109 and the central helix of histone H3 is also required. The observed multiprotein, multisite substrate recognition mechanism among histone modification enzymes provides mechanistic understandings of Rtt109 and Asf1 in H3K56 acetylation, as well as valuable insights into substrate recognition by histone modification enzymes in general.
Organizational Affiliation:
National Laboratory of Biomacromolecules, CAS Center for Excellence in Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, 100101 Beijing, China; University of Chinese Academy of Sciences, 100049 Beijing, China.