Discovery of Small-Molecule Inhibitors of Ubiquitin Specific Protease 7 (USP7) Using Integrated NMR and in Silico Techniques.
Di Lello, P., Pastor, R., Murray, J.M., Blake, R.A., Cohen, F., Crawford, T.D., Drobnick, J., Drummond, J., Kategaya, L., Kleinheinz, T., Maurer, T., Rouge, L., Zhao, X., Wertz, I., Ndubaku, C., Tsui, V.(2017) J Med Chem 60: 10056-10070
- PubMed: 29166018
- DOI: https://doi.org/10.1021/acs.jmedchem.7b01293
- Primary Citation of Related Structures:
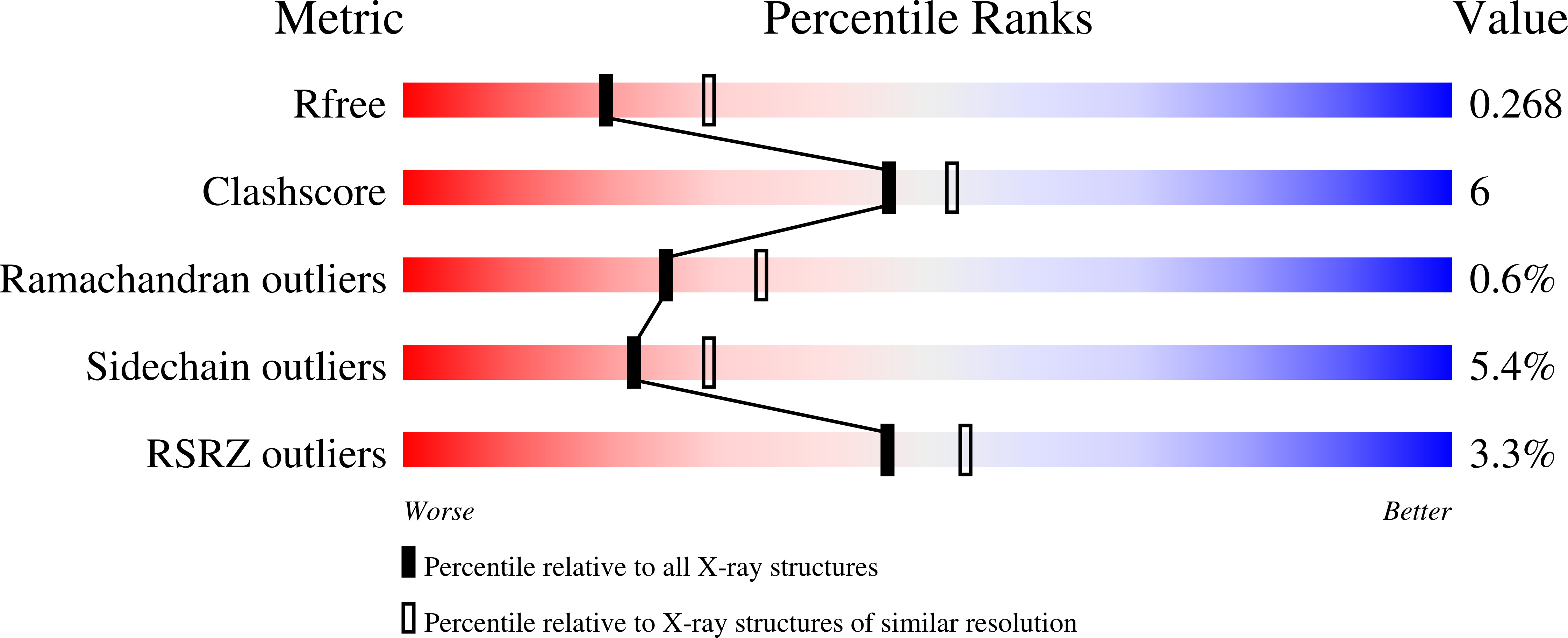
5WHC - PubMed Abstract:
USP7 is a deubiquitinase implicated in destabilizing the tumor suppressor p53, and for this reason it has gained increasing attention as a potential oncology target for small molecule inhibitors. Herein we describe the biophysical, biochemical, and computational approaches that led to the identification of 4-(2-aminopyridin-3-yl)phenol compounds described by Kategaya ( Nature 2017 , 550 , 534 - 538 ) as specific inhibitors of USP7. Fragment based lead discovery (FBLD) by NMR combined with virtual screening and re-mining of biochemical high-throughput screening (HTS) hits led to the discovery of a series of ligands that bind in the "palm" region of the catalytic domain of USP7 and inhibit its catalytic activity. These ligands were then optimized by structure-based design to yield cell-active molecules with reasonable physical properties. This discovery process not only involved multiple techniques working in concert but also illustrated a unique way in which hits from orthogonal screening approaches complemented each other for lead identification.
Organizational Affiliation:
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States.