alpha / beta coiled coils.
Hartmann, M.D., Mendler, C.T., Bassler, J., Karamichali, I., Ridderbusch, O., Lupas, A.N., Hernandez Alvarez, B.(2016) Elife 5
- PubMed: 26771248
- DOI: https://doi.org/10.7554/eLife.11861
- Primary Citation of Related Structures:
5APP, 5APQ, 5APS, 5APT, 5APU, 5APV, 5APW, 5APX, 5APY, 5APZ - PubMed Abstract:
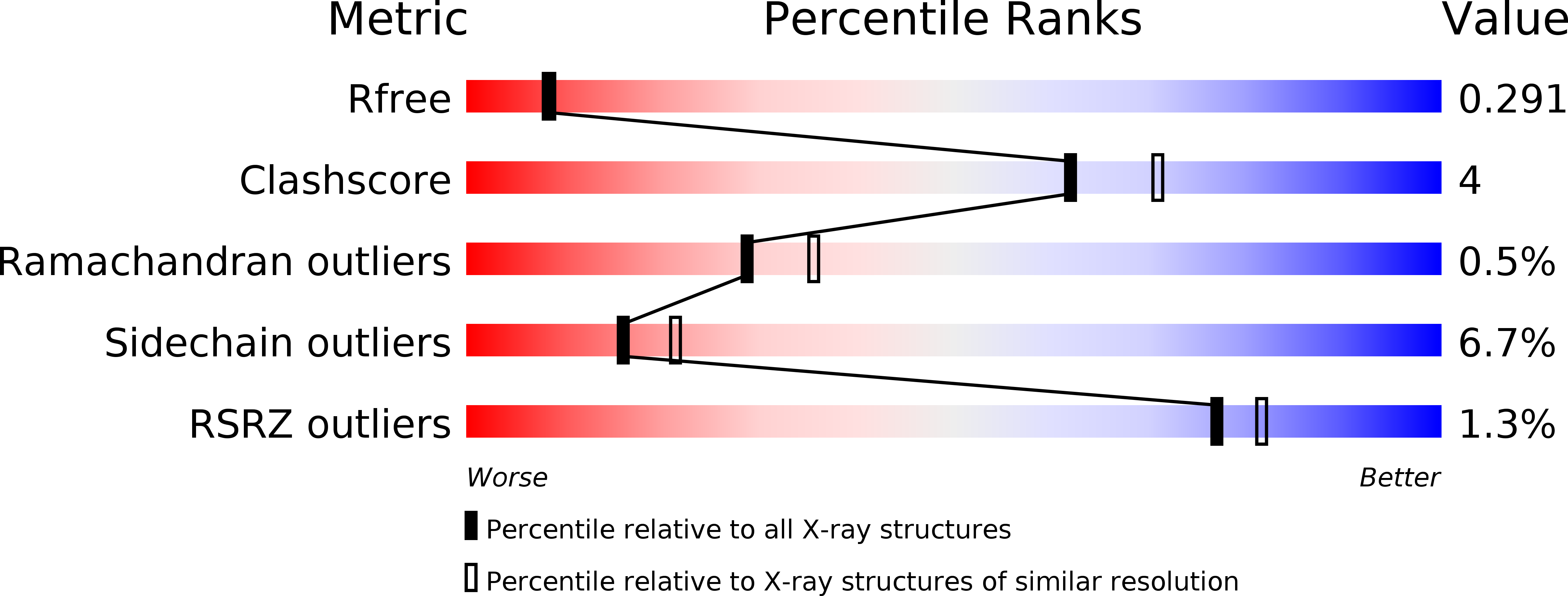
Coiled coils are the best-understood protein fold, as their backbone structure can uniquely be described by parametric equations. This level of understanding has allowed their manipulation in unprecedented detail. They do not seem a likely source of surprises, yet we describe here the unexpected formation of a new type of fiber by the simple insertion of two or six residues into the underlying heptad repeat of a parallel, trimeric coiled coil. These insertions strain the supercoil to the breaking point, causing the local formation of short β-strands, which move the path of the chain by 120° around the trimer axis. The result is an α/β coiled coil, which retains only one backbone hydrogen bond per repeat unit from the parent coiled coil. Our results show that a substantially novel backbone structure is possible within the allowed regions of the Ramachandran space with only minor mutations to a known fold.
Organizational Affiliation:
Department of Protein Evolution, Max Planck Institute for Developmental Biology, Tübingen, Germany.