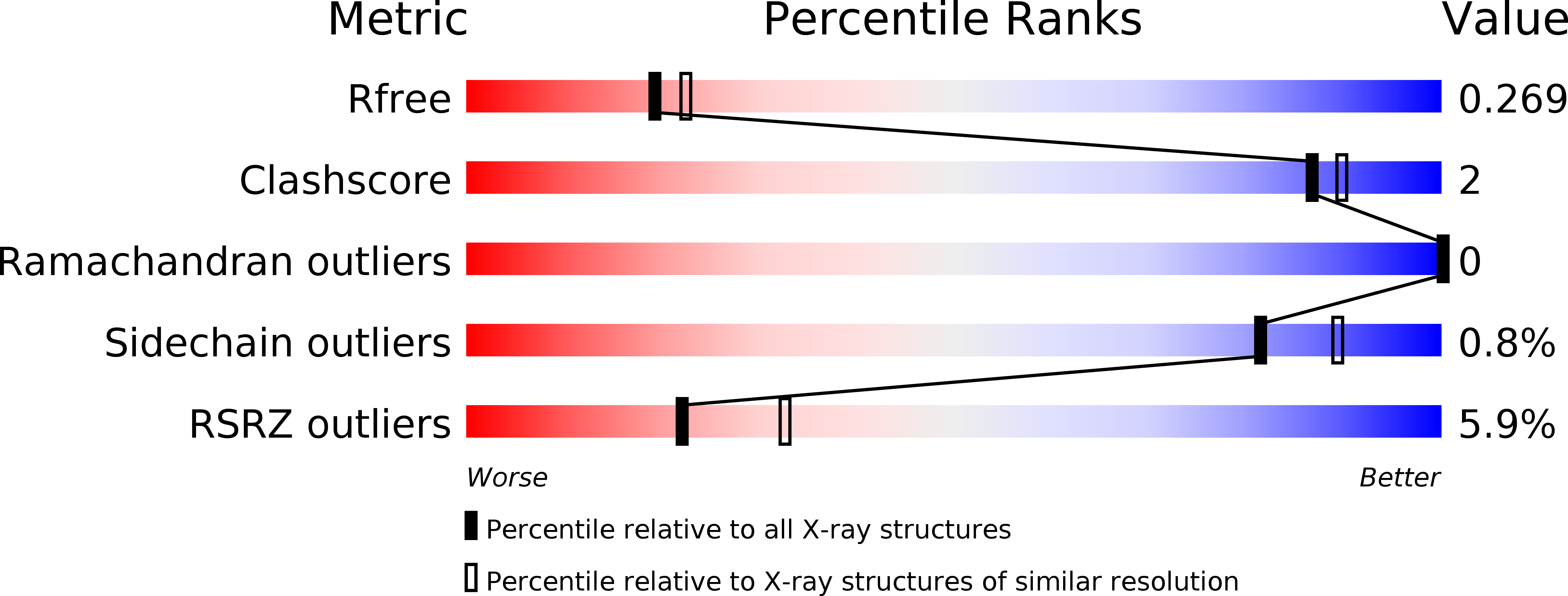
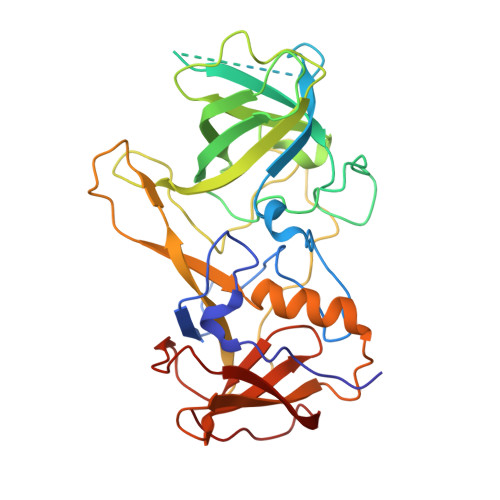
Structural analysis of a feline norovirus protruding domain.
Singh, B.K., Glatt, S., Ferrer, J.L., Koromyslova, A.D., Leuthold, M.M., Dunder, J., Hansman, G.S.(2015) Virology 474: 181-185
- PubMed: 25463616
- DOI: https://doi.org/10.1016/j.virol.2014.10.028
- Primary Citation of Related Structures:
4QUZ, 4QV2, 4QVA, 4QVJ - PubMed Abstract:
Norovirus infects different animals, including humans, mice, dogs, and cats. Here, we show an X-ray crystal structure of a feline GIV.2 norovirus capsid-protruding (P) domain to 2.35Å resolution. The feline GIV.2 P domain was reminiscent of human norovirus P domains, except for a novel P2 subdomain α-helix and an extended P1 subdomain interface loop. These new structural features likely obstructed histo-blood group antigens, which are attachment factors for human norovirus, from binding at the equivalent sites on the feline GIV.2 P domain. Additionally, an ELISA showed that the feline GIV.2 was antigenically distinct from a human GII.10 norovirus.
Organizational Affiliation:
Schaller Research Group at the University of Heidelberg and the DKFZ, Heidelberg 69120, Germany; Department of Infectious Diseases, Virology, University of Heidelberg, Heidelberg 69120, Germany.