Loss of a Functionally and Structurally Distinct ld-Transpeptidase, LdtMt5, Compromises Cell Wall Integrity in Mycobacterium tuberculosis.
Brammer Basta, L.A., Ghosh, A., Pan, Y., Jakoncic, J., Lloyd, E.P., Townsend, C.A., Lamichhane, G., Bianchet, M.A.(2015) J Biol Chem 290: 25670-25685
- PubMed: 26304120
- DOI: https://doi.org/10.1074/jbc.M115.660753
- Primary Citation of Related Structures:
4Z7A, 4ZFQ - PubMed Abstract:
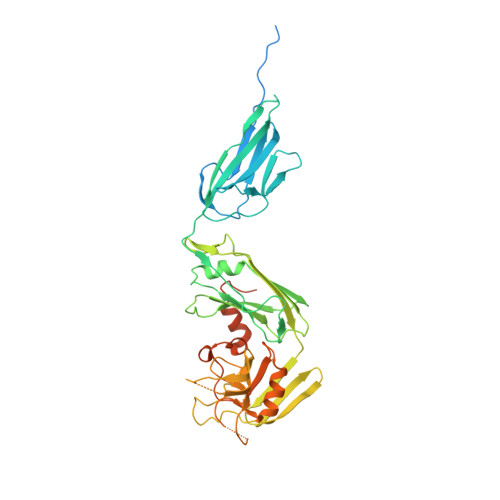
The final step of peptidoglycan (PG) biosynthesis in bacteria involves cross-linking of peptide side chains. This step in Mycobacterium tuberculosis is catalyzed by ld- and dd-transpeptidases that generate 3→3 and 4→3 transpeptide linkages, respectively. M. tuberculosis PG is predominantly 3→3 cross-linked, and LdtMt2 is the dominant ld-transpeptidase. There are four additional sequence paralogs of LdtMt2 encoded by the genome of this pathogen, and the reason for this apparent redundancy is unknown. Here, we studied one of the paralogs, LdtMt5, and found it to be structurally and functionally distinct. The structures of apo-LdtMt5 and its meropenem adduct presented here demonstrate that, despite overall architectural similarity to LdtMt2, the LdtMt5 active site has marked differences. The presence of a structurally divergent catalytic site and a proline-rich C-terminal subdomain suggest that this protein may have a distinct role in PG metabolism, perhaps involving other cell wall-anchored proteins. Furthermore, M. tuberculosis lacking a functional copy of LdtMt5 displayed aberrant growth and was more susceptible to killing by crystal violet, osmotic shock, and select carbapenem antibiotics. Therefore, we conclude that LdtMt5 is not a functionally redundant ld-transpeptidase, but rather it serves a unique and important role in maintaining the integrity of the M. tuberculosis cell wall.
Organizational Affiliation:
From the Taskforce to study Resistance Emergence and Antimicrobial development Technology (TREAT) and Division of Infectious Diseases, The Johns Hopkins University School of Medicine, Baltimore, Maryland 21231.