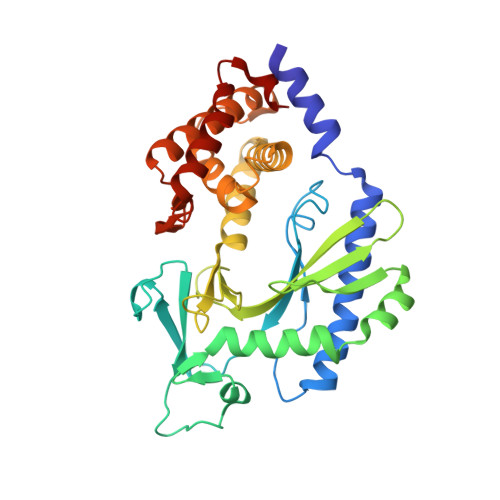
Crystal structure and functional analysis of MiD49, a receptor for the mitochondrial fission protein Drp1.
Loson, O.C., Meng, S., Ngo, H., Liu, R., Kaiser, J.T., Chan, D.C.(2015) Protein Sci 24: 386-394
- PubMed: 25581164
- DOI: https://doi.org/10.1002/pro.2629
- Primary Citation of Related Structures:
4WOY - PubMed Abstract:
Mitochondrial fission requires recruitment of dynamin-related protein 1 (Drp1) to the mitochondrial surface, where assembly leads to activation of its GTP-dependent scission function. MiD49 and MiD51 are two receptors on the mitochondrial outer membrane that can recruit Drp1 to facilitate mitochondrial fission. Structural studies indicated that MiD51 has a variant nucleotidyl transferase fold that binds an ADP co-factor essential for activation of Drp1 function. MiD49 shares sequence homology with MiD51 and regulates Drp1 function. However, it is unknown if MiD49 binds an analogous co-factor. Because MiD49 does not readily crystallize, we used structural predictions and biochemical screening to identify a surface entropy reduction mutant that facilitated crystallization. Using molecular replacement, we determined the atomic structure of MiD49 to 2.4 Å. Like MiD51, MiD49 contains a nucleotidyl transferase domain; however, the electron density provides no evidence for a small-molecule ligand. Structural changes in the putative nucleotide-binding pocket make MiD49 incompatible with an extended ligand like ADP, and critical nucleotide-binding residues found in MiD51 are not conserved. MiD49 contains a surface loop that physically interacts with Drp1 and is necessary for Drp1 recruitment to the mitochondrial surface. Our results suggest a structural basis for the differential regulation of MiD51- versus MiD49-mediated fission.
Organizational Affiliation:
Division of Biology and Biological Engineering, California Institute of Technology, Pasadena, California, 91125.