Human Fyn-SH2 domain in complex with a synthetic high-affinity phospho-peptide
Garcia-Pino, A., Huculeci, R., Lenaerts, T., van Nuland, N.A.J.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Tyrosine-protein kinase Fyn | 122 | Homo sapiens | Mutation(s): 0 Gene Names: FYN EC: 2.7.10.2 | 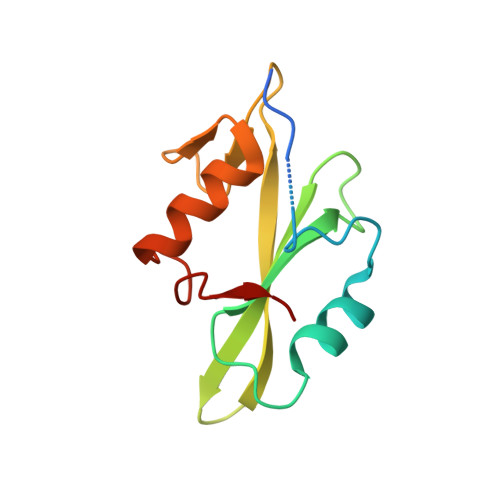 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P06241 (Homo sapiens) Explore P06241 Go to UniProtKB: P06241 | |||||
PHAROS: P06241 GTEx: ENSG00000010810 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P06241 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Middle T antigen | 11 | Alphapolyomavirus mauratus | Mutation(s): 0 | 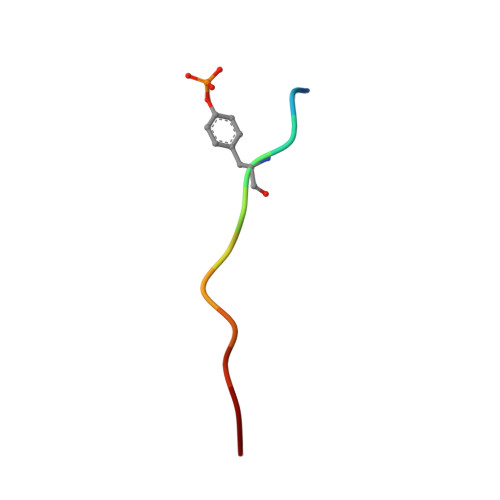 | |
UniProt | |||||
Find proteins for P03079 (Hamster polyomavirus) Explore P03079 Go to UniProtKB: P03079 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P03079 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| 15P Query on 15P | C [auth A] | POLYETHYLENE GLYCOL (N=34) C69 H140 O35 VUYXVWGKCKTUMF-UHFFFAOYSA-N |  | ||
| NA Query on NA | D [auth A] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| PTR Query on PTR | B | L-PEPTIDE LINKING | C9 H12 N O6 P |  | TYR |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 39.248 | α = 90 |
| b = 39.248 | β = 90 |
| c = 145.199 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| PHASER | phasing |