Structural insights into gene repression by the orphan nuclear receptor SHP.
Zhi, X., Zhou, X.E., He, Y., Zechner, C., Suino-Powell, K.M., Kliewer, S.A., Melcher, K., Mangelsdorf, D.J., Xu, H.E.(2014) Proc Natl Acad Sci U S A 111: 839-844
- PubMed: 24379397
- DOI: https://doi.org/10.1073/pnas.1322827111
- Primary Citation of Related Structures:
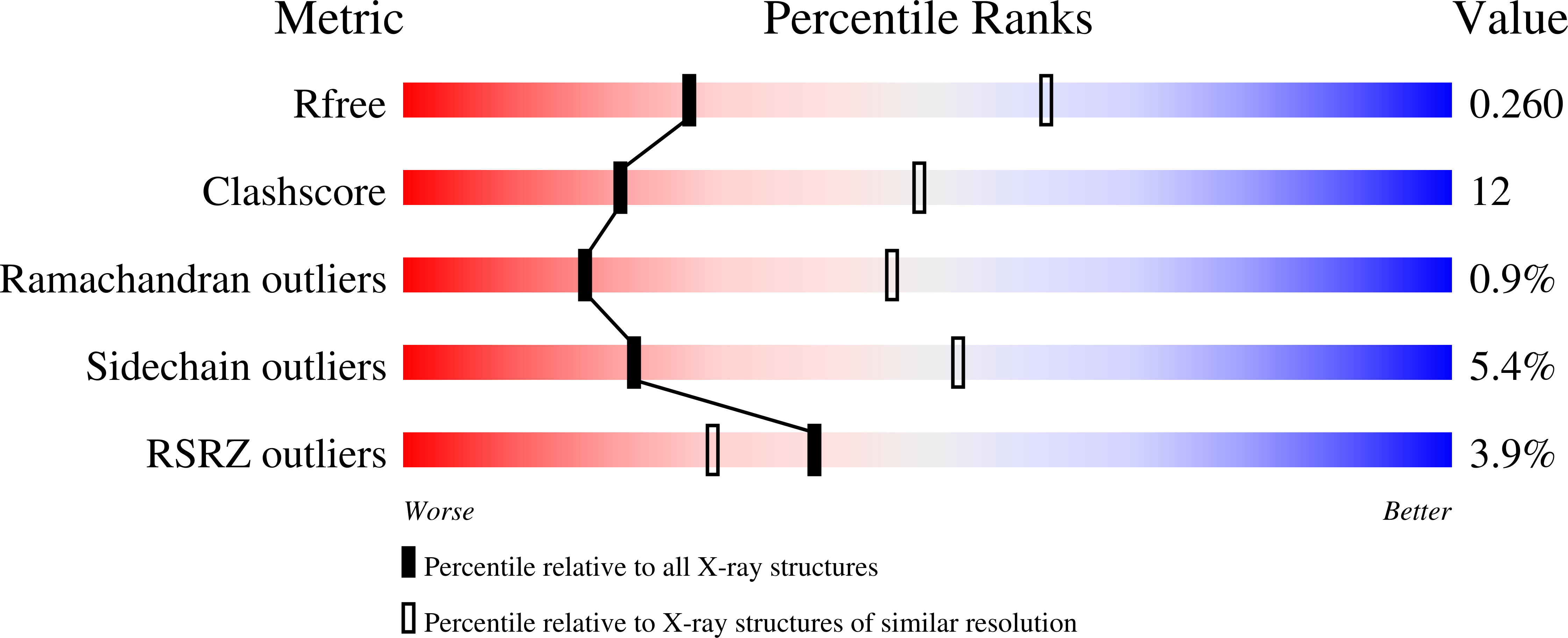
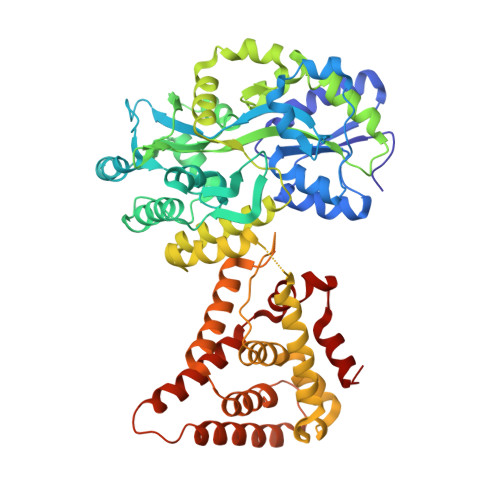
4NUF - PubMed Abstract:
Small heterodimer partner (SHP) is an orphan nuclear receptor that functions as a transcriptional repressor to regulate bile acid and cholesterol homeostasis. Although the precise mechanism whereby SHP represses transcription is not known, E1A-like inhibitor of differentiation (EID1) was isolated as a SHP-interacting protein and implicated in SHP repression. Here we present the crystal structure of SHP in complex with EID1, which reveals an unexpected EID1-binding site on SHP. Unlike the classical cofactor-binding site near the C-terminal helix H12, the EID1-binding site is located at the N terminus of the receptor, where EID1 mimics helix H1 of the nuclear receptor ligand-binding domain. The residues composing the SHP-EID1 interface are highly conserved. Their mutation diminishes SHP-EID1 interactions and affects SHP repressor activity. Together, these results provide important structural insights into SHP cofactor recruitment and repressor function and reveal a conserved protein interface that is likely to have broad implications for transcriptional repression by orphan nuclear receptors.
Organizational Affiliation:
Laboratory of Structural Sciences, Van Andel Research Institute, Grand Rapids, MI 49503.