Structural Insights into the Regulation of ACC2 by Citrate
Kwon, S.J., Cho, Y.S., Heo, Y.S.(2013) Bull Korean Chem Soc 34: 565-568
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
(2013) Bull Korean Chem Soc 34: 565-568
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Acetyl-CoA carboxylase 2 | 573 | Homo sapiens | Mutation(s): 2 Gene Names: ACACB, ACC2, ACCB EC: 6.4.1.2 (PDB Primary Data), 6.3.4.14 (PDB Primary Data) | 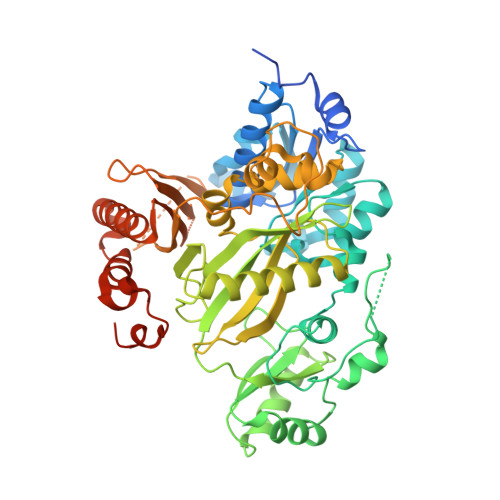 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O00763 (Homo sapiens) Explore O00763 Go to UniProtKB: O00763 | |||||
PHAROS: O00763 GTEx: ENSG00000076555 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O00763 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| SEP Query on SEP | A | L-PEPTIDE LINKING | C3 H8 N O6 P |  | SER |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 75.595 | α = 90 |
| b = 75.595 | β = 90 |
| c = 188.783 | γ = 120 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data collection |
| CNS | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| CNS | phasing |