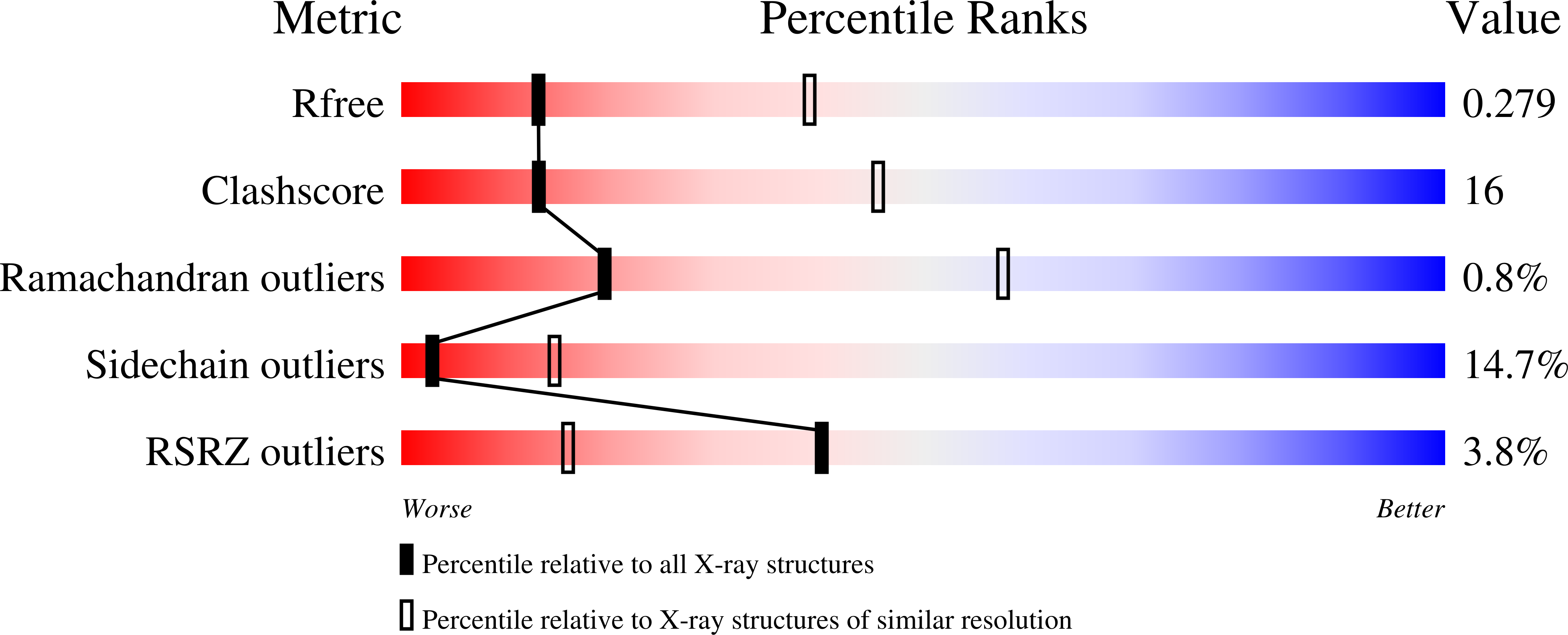
Active site analysis of yeast flavohemoglobin based on its structure with a small ligand or econazole.
El Hammi, E., Warkentin, E., Demmer, U., Marzouki, N.M., Ermler, U., Baciou, L.(2012) FEBS J 279: 4565-4575
- PubMed: 23095020
- DOI: https://doi.org/10.1111/febs.12043
- Primary Citation of Related Structures:
4G1B, 4G1V - PubMed Abstract:
Flavohemoglobins (flavoHbs) serve various microorganisms as the major protective enzymes against NO˙-mediated toxicity. FlavoHbs dominantly function as an NO˙ dioxygenase (O2+ NO→ NO3 -), the required electron being shuttled from NAD(P)H via FAD to the heme iron. The X-ray structures of the flavoHb from Saccharomyces cerevisae presented in complex with an unknown small ligand (Yhb) and with econazole (Yhb(E) ) at 2.1 and 3.0 Å resolutions, respectively, reveal a high architectural accordance between prokaryotic and eukaryotic family members. The active site is characterized by a proximal heme side with a strictly conserved histidine, glutamate and tyrosine triad and a highly variable distal heme side with helix shifts up to 10 Å mainly dependent on the presence/absence and size of the bound ligand. In yeast flavoHb, the small heme iron ligand adjusts a catalytically productive active site geometry that reliably suggests the NO and O(2) binding site. O(2) is activated by its ligation to an electron-rich heme iron and a hydrogen bond to Tyr29 and Gln53. High active site similarities between eukaryotic Yhb and bacterial single-domain globins argue for identical biochemical reactions. Binding of the bulky econazole implies a large-scale induced-fit process concerning, in particular, an outwards shift of helices B and E to increase the active site pocket. Yeast Yhb and Ralstonia eutropha flavoHb both structurally studied in complex with econazole indicate conformational differences between the inhibitors and the polypeptide primarily caused by stable binding of a phospholipid to the latter and by distinct loop D structures.
Organizational Affiliation:
Laboratoire de Chimie Physique, CNRS - Université Paris-Sud, F-91405 Orsay, France.