Structure of the C-terminal domain of the type III effector Xcv3220 (XopL)
Singer, A.U., Schulze, S., Xu, X., Skarina, T., Cui, H., Egler, M., Srikumar, T., Raught, B., Savchenko, A., Bonas, U.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| uncharacterized protein | A [auth B], B [auth A], C | 208 | Xanthomonas euvesicatoria pv. vesicatoria str. 85-10 | Mutation(s): 0 Gene Names: XCV3220 | 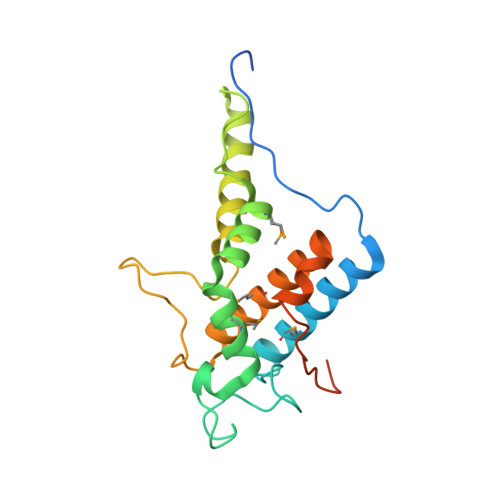 |
UniProt | |||||
Find proteins for Q3BQL2 (Xanthomonas campestris pv. vesicatoria (strain 85-10)) Explore Q3BQL2 Go to UniProtKB: Q3BQL2 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q3BQL2 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| EDO Query on EDO | E [auth B], F [auth B], H [auth A] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| CL Query on CL | D [auth B], G [auth A], I [auth C] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A [auth B], B [auth A], C | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 119.198 | α = 90 |
| b = 119.198 | β = 90 |
| c = 38.679 | γ = 120 |
| Software Name | Purpose |
|---|---|
| HKL-3000 | data collection |
| SHELXCD | phasing |
| PHENIX | refinement |
| HKL-3000 | data reduction |
| HKL-3000 | data scaling |