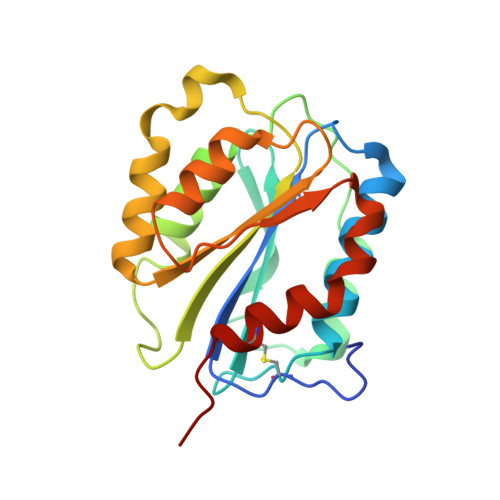
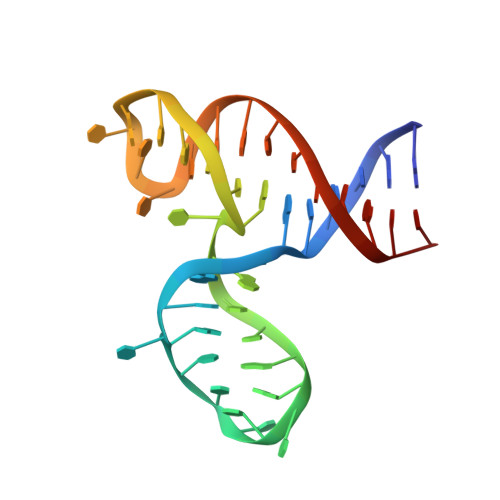
A structural explanation for the antithrombotic activity of ARC1172, a DNA aptamer that binds von Willebrand factor domain A1.
Huang, R.H., Fremont, D.H., Diener, J.L., Schaub, R.G., Sadler, J.E.(2009) Structure 17: 1476-1484
- PubMed: 19913482
- DOI: https://doi.org/10.1016/j.str.2009.09.011
- Primary Citation of Related Structures:
3HXO, 3HXQ - PubMed Abstract:
ARC1172 is a 41-mer DNA aptamer selected to bind the A1 domain of von Willebrand factor (VWF). A derivative of ARC1172 with modifications to increase intravascular survival inhibits carotid artery thrombosis in a Cynomolgus macaque model and inhibits VWF-dependent platelet aggregation in humans, suggesting that such aptamers may be useful to prevent or treat thrombosis. In the crystal structure of a VWF A1-ARC1172 complex, the aptamer adopts a three-stem structure of mainly B-form DNA with three noncanonical base pairs and 9 unpaired residues, 6 of which are stabilized by base-base or base-deoxyribose stacking interactions. The aptamer-protein interface is characterized by cation-pi interactions involving Arg, Lys, and Gln residues, often stabilized by H-bonds with adjacent bases. The ARC1172 binding site on the A1 domain overlaps with that of botrocetin and clashes with glycoprotein Ibalpha binding at an adjacent site, which accounts for the antithrombotic activity of ARC1172 and related aptamers.
Organizational Affiliation:
Department of Medicine, Washington University School of Medicine, St. Louis, MO 63110, USA.