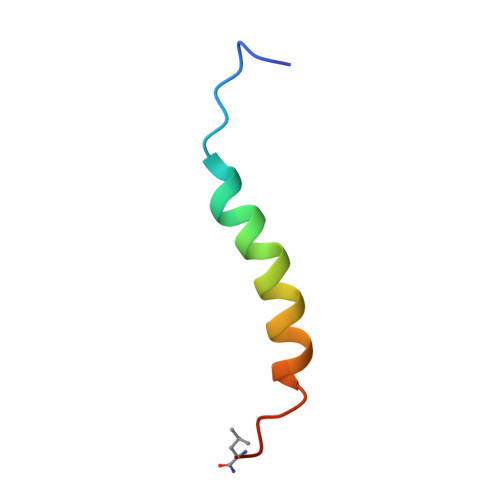
Intrinsic order and disorder in the bcl-2 member harakiri: insights into its proapoptotic activity.
Barrera-Vilarmau, S., Obregon, P., de Alba, E.(2011) PLoS One 6: e21413-e21413
- PubMed: 21731739
- DOI: https://doi.org/10.1371/journal.pone.0021413
- Primary Citation of Related Structures:
2L58, 2L5B - PubMed Abstract:
Harakiri is a BH3-only member of the Bcl-2 family that localizes in membranes and induces cell death by binding to prosurvival Bcl-x(L) and Bcl-2. The cytosolic domain of Harakiri is largely disorder with residual α-helical conformation according to previous structural studies. As these helical structures could play an important role in Harakiri's function, we have used NMR and circular dichroism to fully characterize them at the residue-atomic level. In addition, we report structural studies on a peptide fragment spanning Harakiri's C-terminal hydrophobic sequence, which potentially operates as a transmembrane domain. We initially checked by enzyme immunoassays and NMR that peptides encompassing different lengths of the cytosolic domain are functional as they bind Bcl-x(L) and Bcl-2. The structural data in water indicate that the α-helical conformation is restricted to a 25-residue segment comprising the BH3 domain. However, structure calculation was precluded because of insufficient NMR restraints. To bypass this problem we used alcohol-water mixture to increase structure population and confirmed by NMR that the conformation in both milieus is equivalent. The resulting three-dimensional structure closely resembles that of peptides encompassing the BH3 domain of BH3-only members in complex with their prosurvival partners, suggesting that preformed structural elements in the disordered protein are central to binding. In contrast, the transmembrane domain forms in micelles a monomeric α-helix with a population close to 100%. Its three-dimensional structure here reported reveals features that explain its function as membrane anchor. Altogether these results are used to propose a tentative structural model of how Harakiri works.
Organizational Affiliation:
Centro de Investigaciones Biológicas, Consejo Superior de Investigaciones Científicas, Madrid, Spain.