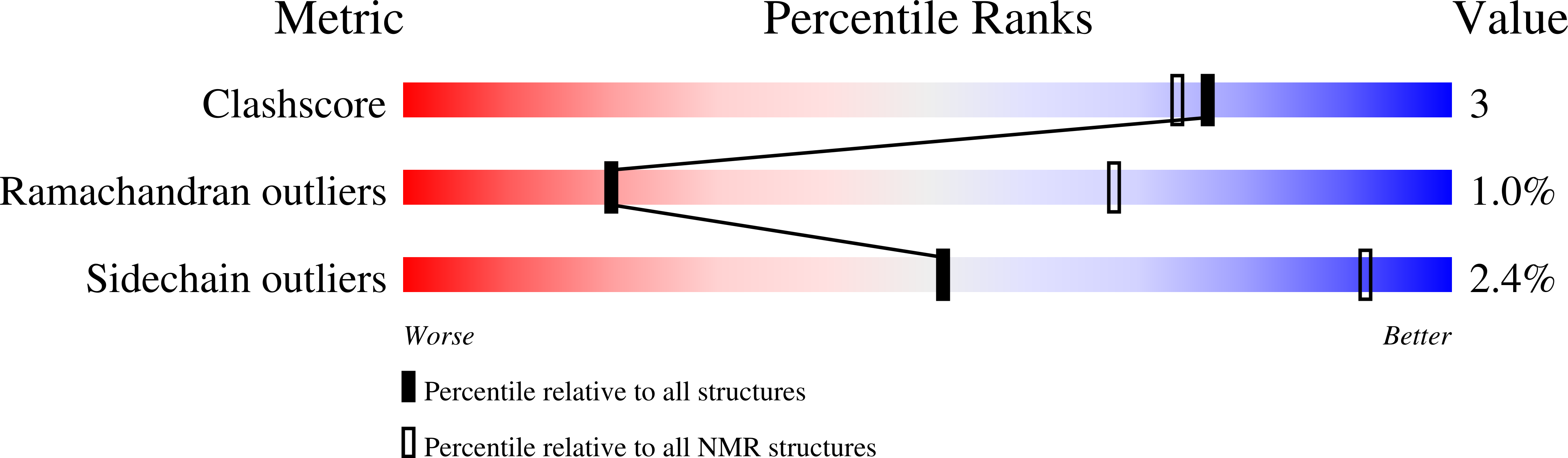Mechanism of the Rpn13-induced activation of Uch37
Jiao, L., Ouyang, S., Shaw, N., Song, G., Feng, Y., Niu, F., Qiu, W., Zhu, H., Hung, L.W., Zuo, X., Eleonora Shtykova, V., Zhu, P., Dong, Y.H., Xu, R., Liu, Z.J.(2014) Protein Cell 5: 616-630
- PubMed: 24752541
- DOI: https://doi.org/10.1007/s13238-014-0046-z
- Primary Citation of Related Structures:
2MKZ - PubMed Abstract:
Uch37 is a de-ubiquitinating enzyme that is activated by Rpn13 and involved in the proteasomal degradation of proteins. The full-length Uch37 was shown to exhibit low iso-peptidase activity and is thought to be auto-inhibited. Structural comparisons revealed that within a homo-dimer of Uch37, each of the catalytic domains was blocking the other's ubiquitin (Ub)-binding site. This blockage likely prevented Ub from entering the active site of Uch37 and might form the basis of auto-inhibition. To understand the mode of auto-inhibition clearly and shed light on the activation mechanism of Uch37 by Rpn13, we investigated the Uch37-Rpn13 complex using a combination of mutagenesis, biochemical, NMR, and small-angle X-ray scattering (SAXS) techniques. Our results also proved that Uch37 oligomerized in solution and had very low activity against the fluorogenic substrate ubiquitin-7-amino-4-methylcoumarin (Ub-AMC) of de-ubiquitinating enzymes. Uch37Δ(Hb,Hc,KEKE), a truncation removal of the C-terminal extension region (residues 256-329) converted oligomeric Uch37 into a monomeric form that exhibited iso-peptidase activity comparable to that of a truncation-containing the Uch37 catalytic domain only. We also demonstrated that Rpn13C (Rpn13 residues 270-407) could disrupt the oligomerization of Uch37 by sequestering Uch37 and forming a Uch37-Rpn13 complex. Uch37 was activated in such a complex, exhibiting 12-fold-higher activity than Uch37 alone. Time-resolved SAXS (TR-SAXS) and FRET experiments supported the proposed mode of auto-inhibition and the activation mechanism of Uch37 by Rpn13. Rpn13 activated Uch37 by forming a 1:1 stoichiometric complex in which the active site of Uch37 was accessible to Ub.
Organizational Affiliation:
National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing, 100101, China.