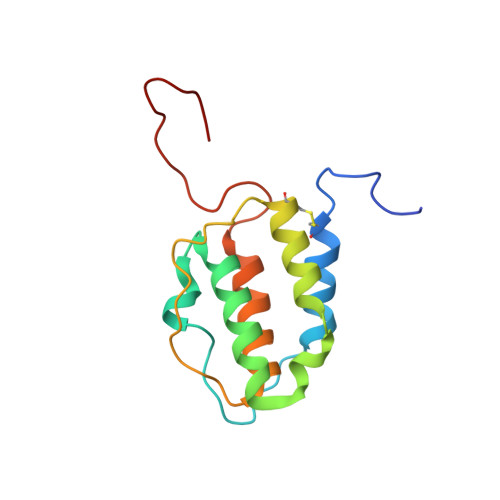
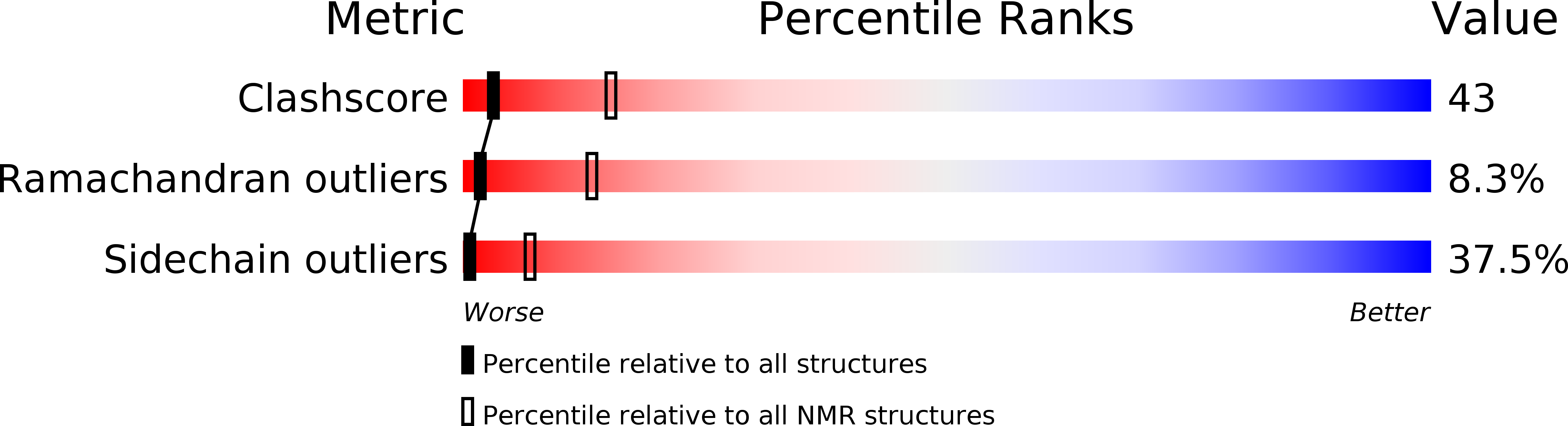Murine interleukin-3: structure, dynamics, and conformational heterogeneity in solution.
Yao, S., Young, I.G., Norton, R.S., Murphy, J.M.(2011) Biochemistry 50: 2464-2477
- PubMed: 21329364
- DOI: https://doi.org/10.1021/bi101810f
- Primary Citation of Related Structures:
2L3O - PubMed Abstract:
Interleukin-3 (IL-3), a cytokine produced primarily by activated T-cells during immune responses, is a crucial regulator of allergic inflammation. The three-dimensional structure of murine IL-3 (mIL-3) has remained elusive owing to its poor solubility and strong tendency toward aggregation under solution conditions typically used for structural studies. Here we describe the solution properties and structure of mIL-3 determined by NMR using an engineered construct of mIL-3 (mIL-3(33-156)). mIL-3 adopts a four-helical bundle fold, typical of proteins belonging to the short-chain cytokine family, and features a core of highly conserved hydrophobic residues. While significant line broadening and peak disappearance were observed in NMR spectra at higher temperatures, there was no evidence for temperature-dependent changes of the oligomeric state of mIL-3(33-156). Further analysis of the temperature dependence of amide (1)H chemical shifts and backbone (15)N relaxation parameters, including (15)N relaxation dispersion, revealed the presence of significant conformational exchange and local conformational heterogeneity. Residues recently shown by mutagenesis to play key roles in β(IL-3) receptor recognition and activation, which are located within the α(A) and α(C) helices and aligned on one face of the mIL-3(33-156) structure, are relatively rigid. In contrast, pronounced conformational heterogeneity was observed for a cluster of residues located on the opposite side of mIL-3, which corresponds spatially to sites in the related cytokines human IL-3, IL-5, and GM-CSF that are known to mediate interactions with their respective α-receptor subunits. Such conformational heterogeneity may facilitate the interaction of mIL-3 with each of two naturally occurring mIL-3Rα isoforms, leading to structurally distinct high-affinity complexes.
Organizational Affiliation:
The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, Victoria 3052, Australia. syao@wehi.edu.au