The Pub Domain Functions as a P97 Binding Module in Human Peptide N-Glycanase.
Allen, M.D., Buchberger, A., Bycroft, M.(2006) J Biol Chem 281: 25502
- PubMed: 16807242
- DOI: https://doi.org/10.1074/jbc.M601173200
- Primary Citation of Related Structures:
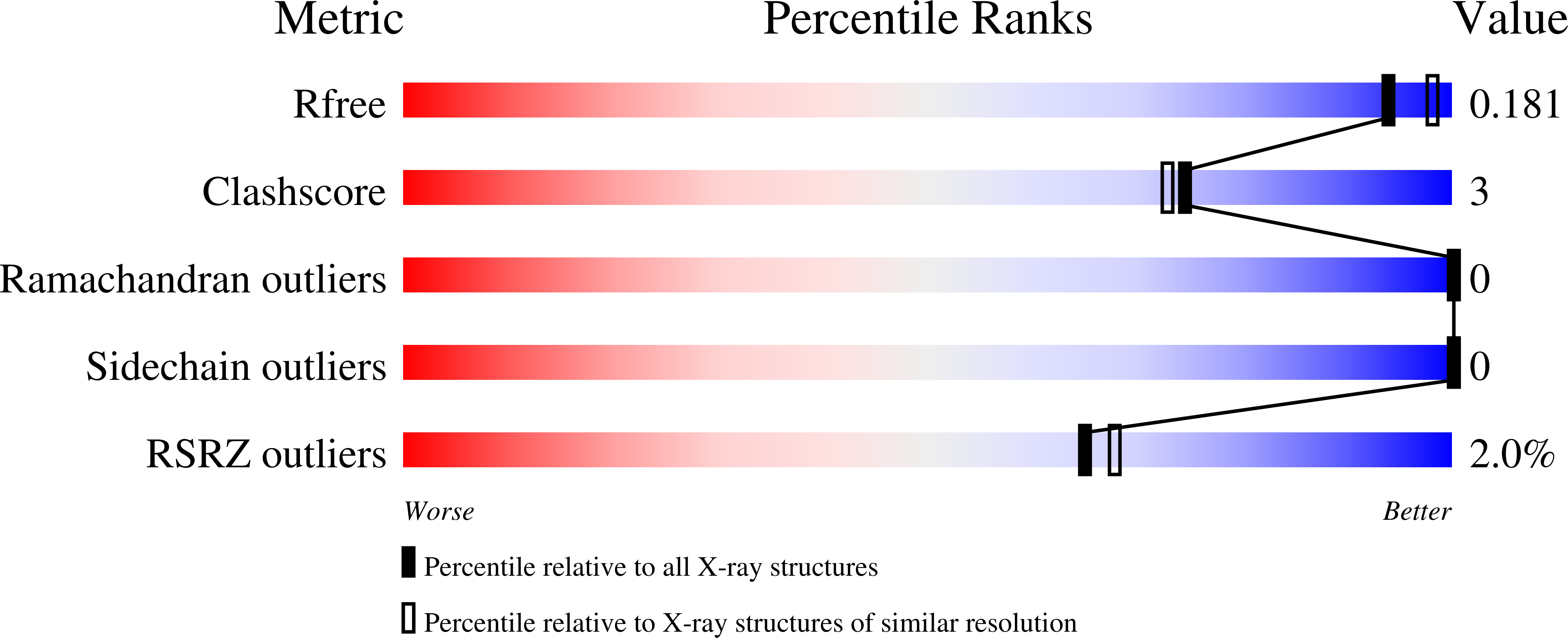
2CCQ, 2CM0 - PubMed Abstract:
The AAA ATPase p97 is a ubiquitin-selective molecular machine involved in multiple cellular processes, including protein degradation through the ubiquitin-proteasome system and homotypic membrane fusion. Specific p97 functions are mediated by a variety of cofactors, among them peptide N-glycanase, an enzyme that removes glycans from misfolded glycoproteins. Here we report the three-dimensional structure of the aminoterminal PUB domain of human peptide N-glycanase. We demonstrate that the PUB domain is a novel p97 binding module interacting with the D1 and/or D2 ATPase domains of p97 and identify an evolutionary conserved surface patch required for p97 binding. Furthermore, we show that the PUB and UBX domains do not bind to p97 in a mutually exclusive manner. Our results suggest that PUB domain-containing proteins constitute a widespread family of diverse p97 cofactors.
Organizational Affiliation:
Centre for Protein Engineering, Medical Research Council, Hills Road, Cambridge CB2 2QH, United Kingdom.